Manipulated Cells Can Rebuild Damaged Tissue, Says West
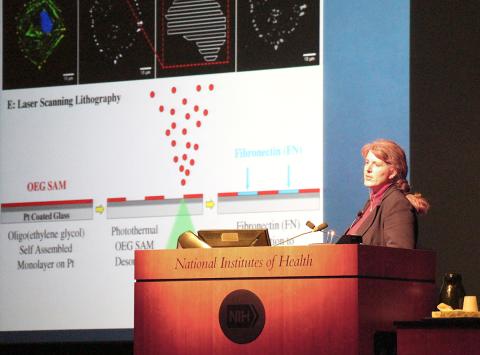
Photo: Raymond Macdougall
Biomaterials expert Dr. Jennifer West is a leader in her cutting-edge field, a founding inventor at two companies, holder of 20 biotechnology patents and the Fitzpatrick family university professor of engineering and associate dean for Ph.D. education at Duke University’s Pratt School of Engineering. She spoke Mar. 22 at the NIH Director’s Wednesday Afternoon Lecture Series.
West’s talk focused on work in her lab to design biomaterials for regenerative medicine, a field that includes tissue engineering and research on self-healing, where the body uses its own systems, sometimes with help from foreign biological material, to recreate cells and rebuild tissues and organs. She described several projects that use regenerative medicine principles to create controlled environments for cells where researchers can manipulate cell behaviors.
From 2006 to 2009, West was part of a team that received the first Quantum Grant from NIH’s National Institute of Biomedical Imaging and Bioengineering. Quantum Grants support research projects that target solutions to a major medical or public health challenge through technological innovation. The project investigated ways to regenerate damaged brain cells and blood vessels for the treatment of stroke.
Since that time, West has been a principal or co-investigator on a range of NIH-funded projects that continue to push the envelope with ever more sophisticated techniques, moving into the realm of bioprinting—first in 2-D and then with 3-D printing. Of her current work with cell regeneration, West said, “It’s not just a cell’s identity, but how it is spatially localized and organized, which controls the signals for cell differentiation, proliferation and other behaviors.”
Among the technologies featured in her lecture, West described bioprinting in hydrogel layers with strategically positioned, nano-scale chemical switches, some that are turned on with laser optics. The switches send cellular signals at times and at locations that are critical in forming cell material. The approach uses cues from nature that can mimic some of the functions and spatial relationships of the extracellular matrix—the structural and biochemical support to the surrounding cells.
“You can take images from vasculature of lots of different tissues that give us different geometries and chemo-dynamics—and then generate those same patterns in hydrogels,” West explained. The patterns resemble the capillaries on the underside of a leaf, but each is distinct, to mimic the vasculature of such tissues as heart, brain and the retina.
“This is an exciting time, where cell biology is advancing, providing a lot more information about how to design systems to get the best possible tissue engineering and medicine outcomes,” West said. “But where engineering is advancing we can really contribute a lot to tools that help us develop a better understanding of cell biology. Hopefully, as we move forward we’ll see the iterative interactions between those two fields and reap the benefits that can come from engineering and biology working together.”
