FDA-Approved Drug Effectively Treats Rare Chronic Immune Disorder
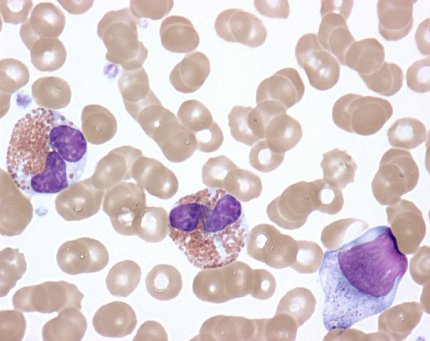
Photo: NIAID
A drug approved to treat a severe form of asthma dramatically improved the health of people with rare chronic immune disorders called hypereosinophilic syndromes (HES) in whom other treatments were ineffective or intolerable. This finding comes from a small clinical trial led by scientists at NIAID and conducted through a partnership with the global biopharmaceutical company AstraZeneca. The results were published online Apr. 3 in the New England Journal of Medicine.
“People living with a rare disease often have few, if any, effective treatment options,” said NIAID director Dr. Anthony Fauci. “This promising treatment advance for people with hypereosinophilic syndromes is just one example of how NIH research responds to the unique medical needs of individuals with rare diseases.”
HES is caused by higher-than-normal numbers of white blood cells called eosinophils in the blood, tissues or both. While most people have 0 to 500 eosinophils per microliter (ųL) of blood, people with HES typically have more than 1,500 eosinophils/ųL. The symptoms of HES vary widely from one patient to the next and can affect the heart, lungs, skin, gastrointestinal tract, central nervous system and other organ systems.
Nearly all existing therapies for HES involve drugs that are not specifically approved for treating the syndromes, have significant side effects and sometimes become less effective over time. This study was only the second randomized, placebo-controlled trial—the gold standard of medical research—to test the effectiveness of a drug specifically for treating HES. The trial was led by Dr. Amy Klion, chief of the human eosinophil section in NIAID’s Laboratory of Parasitic Diseases.
