Father of Cancer Immunotherapy
Rosenberg’s Research Spans a Half-Century and Counting

About 55 years ago, Dr. Steven Rosenberg had a hunch that a patient’s own immune system could help fight cancer. Since arriving at NIH in 1974, he has painstakingly pursued this line of inquiry with some groundbreaking results.
In the beginning, his hunch diverged from all scientific literature. “It was hypothetical—no one had ever demonstrated an immune manipulation that could cause cancers to disappear,” said Rosenberg, chief of surgery at the National Cancer Institute (NCI), a position he has held for 49 years.
His research journey was inspired by two unusual cases early in his career.
In 1968, while a surgical resident at Brigham Hospital in Boston, Rosenberg was tasked with removing the gallbladder of a 63-year-old patient. The patient’s chart revealed he was hospitalized 12 years earlier with stomach cancer that had spread to the liver. Nothing could be done; no treatment was given.
“When I operated on him, he had no cancer,” recounted Rosenberg. “He had undergone one of the rarest events in all of medicine, a spontaneous regression of his metastatic cancer.”
A few years earlier, a patient at Brigham had developed widespread kidney cancer following an organ transplant. It turned out, the kidney he’d received was cancerous. When the immunosuppressive drugs—given to prevent organ rejection—were stopped, his body rejected the kidney and, incredibly, his cancer disappeared.
“That told me if you could spark a strong enough immune reaction, you could get large, vascularized cancers to disappear,” Rosenberg said. “That then set me on the path these past 50 years to try to figure out how that happened, and how to make it happen more often.”
An Aspiring Cowboy

Born and raised in the Bronx, New York, as a kid, Rosenberg wanted to be a cowboy. But that aspiration quickly turned to medicine.
Stories from his parents, Jewish immigrants who fled Poland, moved him deeply. Many of his relatives perished in the Holocaust. His family’s history instilled in him a lifelong desire to end needless suffering.
After medical school, Rosenberg paused his surgical residency to pursue a Ph.D. in biophysics at Harvard. He then conducted lab research, including a stint as an NCI immunology fellow, before finishing his residency and coming to work permanently at NCI as a clinician-scientist.
Along the way, there were false leads and failed experiments. In many cases, the cancer won.
“Most things don’t work,” as is often the plight of any clinical research, noted Rosenberg. “The overwhelming majority of experiments do not work in ways, and give the kinds of results, that we seek.”
But he persevered. “Cancer is a devastating disease. It’s a holocaust,” he said. “Patients who are innocent develop problems they cannot control, while their families sit by impotently and watch them suffer and ultimately die of the disease. It’s horrible. When you see that happening, as a doctor, you feel you have to do something about this. And that’s something that has kept me going for a very long time.”
Rosenberg ultimately became a cowboy, in a sense, exploring uncharted territory, pushing boundaries, all the while seeking to rein in cancer.
Studying Immune Warriors

Rosenberg began his quest to find an immune response to cancer by studying T lymphocytes—the body’s immune warriors. But these white blood cells don’t live long outside the body. Other researchers were publishing studies with interleukin-2 (IL-2)—proteins made by white blood cells (leukocytes) that facilitate growing T cells [a type of immune cell] outside the body.
“I realized perhaps we could get anti-tumor lymphocytes to grow inside the body,” he said. “So I started administering IL-2 to treat patients.”

It was a discouraging time, recalled Rosenberg. His team treated 66 patients in a row without success. Ongoing research showed IL-2 had a short half-life in the body of only about eight minutes. It needed to be given repeatedly in higher doses.
Then, a breakthrough. His 67th patient, Linda Taylor, received the right combination and her metastatic melanoma disappeared.
“Melanoma was previously a universally lethal disease once it had spread,” Rosenberg said. “Linda was treated in 1984 and is still alive and disease-free today.”

Subsequent large studies by Rosenberg’s group showed that IL-2 could induce cancer regression in 15-20% of patients with metastatic melanoma and kidney cancer. IL-2 became the first Food and Drug Administration (FDA)-approved immunotherapy for cancer in the 1990s.
Rosenberg’s team then discovered that lymphocytes that exist inside a tumor can recognize the cancer. The tumor is a sink for these immune cells, which they named “tumor-infiltrating lymphocytes” (TILs) and began administering TILs to patients.
“That was the first direct demonstration that lymphocyte transfer could cause tumor regression,” Rosenberg said. “That was developed to the point where, in our latest trials of almost 200 patients, 56% underwent a cancer regression and a quarter of patients with metastatic melanoma would undergo complete—what appeared to be durable—regressions. Now, we’re talking about response rates in the 50% range, not the 15% range of IL-2.”
Going Down Two Research Paths
Building on many years of study and clinical trials, Rosenberg’s research went in two directions. One is continuing to use a patient’s own natural lymphocytes that can recognize cancer as a living drug. The second is to genetically modify immune cells to target cancer. Rosenberg was, in fact, the first person ever to insert foreign genes into a human, paving the way for the field of gene therapy.
Both types of cancer immunotherapy “are what we’re trying to improve now to fight the common solid cancers [that begin in major organs] for which there are no curable treatments, short of surgery, once they have spread.” Solid cancers account for 90% of all cancer deaths.
“It turns out, lymphocytes were recognizing the DNA mutations that cause the cancer,” distinguishing malignant cells from normal ones, Rosenberg said. “That’s probably the final common pathway of all immunotherapies…It’s somewhat ironic that the very gene mutations that cause the cancer will turn out to be its Achilles heel, to target it.
“We developed techniques to specifically study the mutation products that T cells recognized and have now developed that into a whole field of study,” he said. Initially, he focused on naturally occurring T cells.

When Rosenberg first proposed inserting genetically modified immune cells into patients, he not surprisingly faced major opposition—“it’s too risky” and “it’s immoral” were among the main arguments. When review board approval came, in 1984, the first patient received his own lymphocytes modified by a bacterial gene not as treatment, but to see where they went in the body.
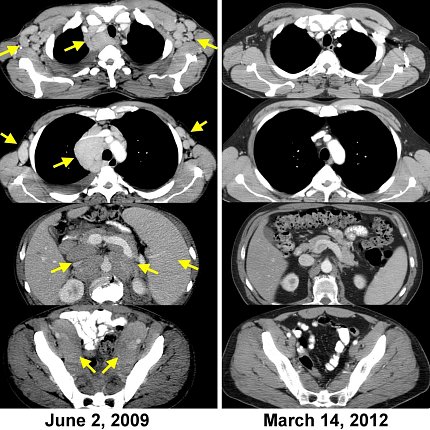
That led his team to identify the actual lymphocyte receptors that recognize the cancer and—years later, working with Dr. James Kochenderfer, a fellow in Rosenberg’s lab—gave rise to CAR [chimeric antigen receptor] T-cell therapy—a way to modify lymphocytes to attack B-cell malignancies such as lymphoma and leukemia.
“We treated the first patient ever to receive these CAR-T cells in 2009. He underwent a complete regression and is disease-free more than 12 years later,” Rosenberg said.
Scaling Up
One of Rosenberg’s former fellows started a company to commercialize these new lab creations known as CAR-T cells, signing a cooperative research agreement with NCI. Five years later, it was sold in a multi-billion-dollar deal to Gilead Sciences.
“It’s a proud example of how findings made in government institutions can find their way out into the world,” Rosenberg said.
This process involves making retroviruses that insert and integrate appropriate genes into the patient’s normal T cells, growing them to large numbers and infusing them back into the patient. Now, NCI works with several pharmaceutical companies, with FDA approval, to develop cell transfer immunotherapies for patients with common solid cancers.
Creating Tomorrow’s Medicine

Photo: Rhoda Baer/NCI
Rosenberg said one of the biggest challenges throughout his career has been understanding the underlying, complicated biology of cancer—the complexity of tumor recognition; identifying cells that cause cancer progression; understanding and overcoming treatment resistance.
“We study basic science and then try to translate it to patients,” he said. “That’s what NIH does. We practice the best of today’s medicine, but we’re here to create tomorrow’s medicine.”
There still is a desperate need to supplement the three existing forms of cancer treatments: surgery, radiation and chemotherapy.
“We now have this three-legged stool a lot more stable with the addition of this fourth leg,” Rosenberg concluded. “Immunotherapy has a lot of promise for developing more effective cancer treatments.”
Explore more innovations across NIH in our NIH Makers series.
