Multiple ‘ACTTs’
NIAID Researcher Discusses Outcomes of Covid Antiviral Studies

Researchers knew they had to act fast. Alarmed at the first reports of a quickly spreading new coronavirus in early January 2020, NIAID researchers immediately began planning for clinical trials to test promising therapeutics even before there were known Covid-19 cases in the U.S.
“Our initial focus was getting the vaccine study started but we quickly started planning [a clinical trial for the antiviral remdesivir] as we saw more and more cases,” said Dr. John Beigel at a Covid-19 Scientific Interest Group virtual lecture earlier this year.
Beigel, associate director for clinical research in NIAID’s Division of Microbiology and Infectious Diseases, has been instrumental in NIH’s research response to respiratory viruses for nearly 20 years. Last year, he led the team implementing the Adaptive Covid-19 Treatment Trial (ACTT), the first large U.S. Covid-19 study launched after the first U.S. patients were identified.
On Jan. 31, 2020, Beigel and colleagues submitted a synopsis of a trial design to the FDA. Three weeks later, the first ACTT site was activated. In less than a year, four phases of ACTT were launched to study the leading antiviral and anti-inflammatory candidates in hospitalized Covid-19 patients.
The effort required pooling resources and identifying sites of high epidemiology across the country and around the world. Enrollment for ACTT-1 started slowly, then rapidly expanded, mirroring the Covid trajectory across the country, recounted Beigel.

Photo: Manjurul/istock/getty
In 58 days, the trial had enrolled 1,062 people at 71 sites across 10 countries. Conducted at sites across the U.S., Europe and Asia, the results from ACTT-1 led to the licensing of the antiviral remdesivir and ACTT-2 supported FDA’s emergency use authorization of the anti-inflammatory baricitnib.
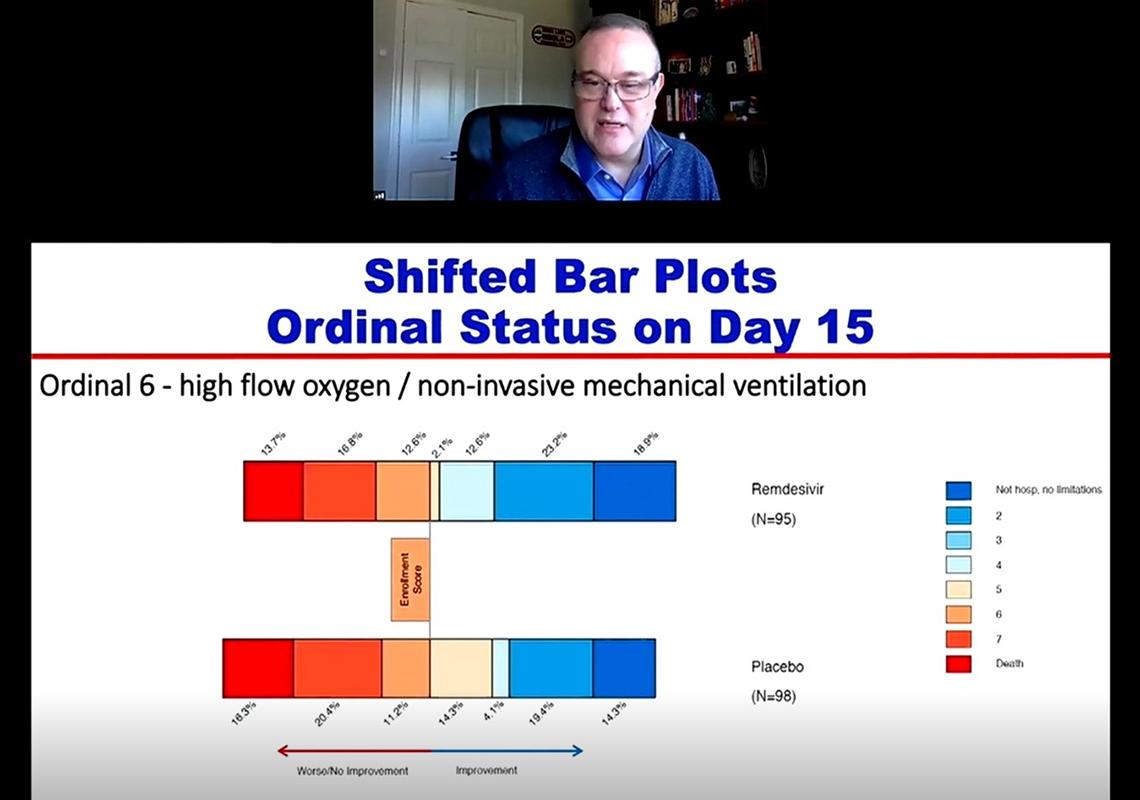
In ACTT-1, participants received remdesivir or a placebo for 10 days. The endpoint of the 28-day study was the time to recovery or to improved clinical status. On average, the time to recovery for patients on remdesivir went from 15 days down to 10. The antiviral showed greater efficacy in people with more advanced disease, with the most benefit seen in those patients on oxygen. The study, however, did not demonstrate a benefit to mortality.
“Is remdesivir effective for treating somebody hospitalized with Covid-19? I think the answer has to be yes—faster recovery, [fewer] days on oxygen, less time on vent—[means fewer] resources that may be strained during a pandemic,” said Beigel. “We see it out in California, when they’re talking about oxygen pipes freezing because there’s so much oxygen flowing through them, because everyone’s on oxygen. If [we] can get people out of the hospital, off oxygen faster, that turns into tangible results.”
Photo: NIAID
The World Health Organization, however, came to different conclusions about remdesivir in its Solidarity trial. The study—involving 12,000 patients hospitalized for Covid-19 at 500 hospitals in 30 countries—tested 4 repurposed antivirals, including remdesivir and hydroxychloroquine. The WHO concluded that none of these antivirals lessened duration of hospital stay or improved mortality rates.
But there were limitations and biases in this trial, argued Beigel, including lack of a placebo group. “There are no trial results free from uncertainty,” he said. “Bigger trials are good; bigger trials with more uncertainty are not necessarily better.”
By spring 2020, NIAID launched ACTT-2, enrolling 1,033 hospitalized adults at 71 sites, to test whether combining remdesivir with the anti-inflammatory baricitnib would improve recovery time and reduce mortality. Investigators chose baricitnib for its safety profile and wide availability worldwide. What’s more, it has potential antiviral effects.
“For ACTT-2, I’d say baricitnib in addition to remdesivir improves outcomes,” Beigel said. “It improves time to recovery. Those with high- or low-flow oxygen appear to have the largest benefit.”
In ACTT-1, many in the high-oxygen group didn’t significantly improve on remdesivir alone; ACTT-2 had the largest benefits in that group. But in both ACTTs, there still was little to no improvement in those on mechanical ventilation.
Scientists at the University of Oxford found hopeful news for this critically ill group. Their Recovery trial revealed that dexamethasone, a corticosteroid, improved outcomes for people with severe Covid infections. Mortality decreased in the oxygen therapy groups, but the biggest benefit was seen in the mechanical ventilation group. This ongoing trial also is studying several antivirals including two repurposed HIV drugs, lopinavir and ritonavir, that have not shown benefit against Covid. Remdesivir has not, so far, been studied in the Recovery trial.
From August to November, there was an ACTT-3, to test the antiviral interferon, though the results are not yet available. In December, ACTT-4 began enrolling a 1,500-patient cohort to study whether dexamethasone or baricitnib work better when paired with remdesivir among hospitalized patients on oxygen and/or noninvasive ventilation.
It’s tough to compare results between ACTT, Solidarity and Recovery, said Beigel, because the design, parameters and populations are not analogous. Even mortality rates aren’t comparable between the different trials.
“A drug that has a detectable effect in a population with a high risk of death may not have the same effect once you get to a lower [risk] of death,” he said.
Instead, he advised considering the totality of the data and reaping the lessons from each trial, which offer important guidance for specific populations. Additionally, standard of care has improved over time which, on its own, is improving outcomes.
Researchers worldwide continue studying ways to reduce illness severity and mortality. To date, there is no fully effective treatment for Covid-19. “There’s still a lot of work to be done,” said Beigel. This is the value of complex trials, he continued, generating datasets and samples to permit analysis and testing to better inform how to best treat Covid-19.
