Researchers Discover Biomarker for Tracking Depression Recovery
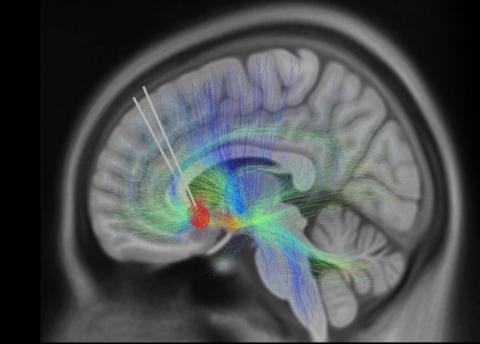
Photo: KI SEUNG CHOI/MAYBERG LAB, ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI
Using a novel deep brain stimulation (DBS) device capable of recording brain signals, researchers have identified a pattern of brain activity or “biomarker” related to clinical signs of recovery from treatment-resistant depression. Findings from this small study are an important step toward using brain data to understand a patient’s response to DBS treatment.
The study was published in Nature and supported by NIH’s Brain Research Through Advancing Innovative Neurotechnologies, or the BRAIN Initiative.
Although the approach is still experimental, clinical research shows that DBS can safely and effectively treat cases of treatment-resistant depression, in which symptoms have not improved with antidepressant medications. People receiving DBS undergo surgery to have a thin metal electrode implanted into specific brain areas to deliver electrical impulses that modulate brain activity.
The small study enrolled 10 adults with treatment-resistant depression, all of whom underwent DBS therapy for six months. Each participant received the same stimulation dose to begin and then stimulation levels were increased once or twice.
Later, researchers used artificial intelligence (AI) tools to analyze collected brain data from six patients and observed a common brain activity signature or biomarker that correlated with patients self-reporting feeling symptoms of depression or stable as they recovered. In one patient, researchers identified the biomarker and were retrospectively able to predict that a patient would fall back into a major depressive episode four weeks before clinical interviews showed they were at risk of a relapse occurring.
The patients in the study responded well to DBS therapy; after six months, 90% showed a significant improvement in depression symptoms and 70% were in remission or no longer depressed.
“This study demonstrates how new technology and a data-driven approach can refine DBS therapy for severe depression, which can be debilitating,” said Dr. John Ngai, director of the BRAIN Initiative. “It’s this type of collaborative work that moves promising therapies closer to clinical use.”
