30 Years of Lupus Research Lauded

Photo: Marleen Van Den Neste
Researchers, patients, advocates and NIH staff gathered recently to celebrate the 30th anniversary of lupus clinical research within the lupus natural history protocol at NIH, marking key accomplishments while looking toward the future.
As Dr. Sarfaraz Hasni, chief of the lupus clinical trials unit at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), indicated, the event’s colorful purple poster adorned with butterflies in different shapes and sizes aptly characterizes NIH’s work on the condition.
“Each and every patient is individually different,” he explained of the poster’s inspiration, like “butterflies that are all different shapes and sizes.”
Hasni also recognized the “unwavering NIH support to researching conditions like lupus,” a sustained effort that involves multiple institutes at the agency.

Photo: Marleen Van Den Neste
The May 31 event, held in Lipsett Amphitheater, was an opportunity to recognize the advances in lupus research since the inception of the D.C. Lupus Consortium.
“The D.C. Lupus Consortium is a very important catalyst for lupus research,” said NIAMS Director Dr. Lindsey Criswell, “and we thank you for your important role in expanding our lupus research efforts.”
The event included short presentations from a variety of NIH and NIH-supported researchers and other experts active in the field, testimonials from patients who have participated in NIH lupus clinical trials and reflections from patient advocacy groups.
Major themes included how lupus presents differently in different patients, the promise of personalized treatment and the need to ensure that patients are involved in the design and execution of any research.
Women get lupus about nine times more often than men and it is also more common among African Americans and people of American Indian and Asian descent. Approximately 39% of those diagnosed with lupus are African American, whereas approximately 36% are White. Ensuring that all people are adequately represented in clinical trials is key to developing treatments that work for everybody.
“The more we learn about lupus patients anywhere, the more we learn about lupus patients everywhere,” said Dr. Laura Lewandowski, assistant clinical investigator and head of NIAMS’s lupus genomics and health disparities unit, in her discussion about understanding the genetics of lupus in global populations.
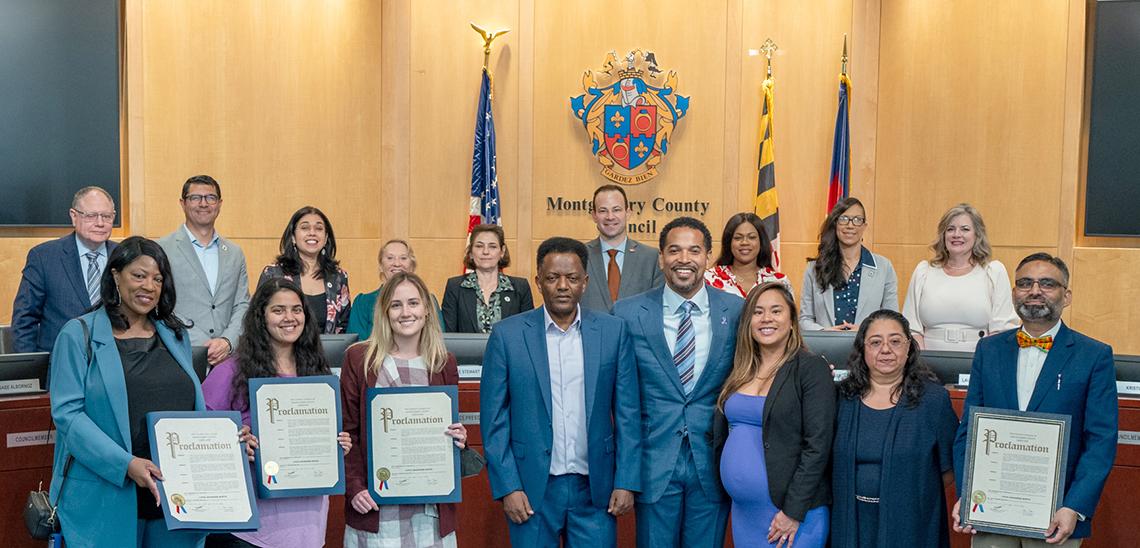
Photo: BENJAMIN SKY BRANDT/MONTGOMERY COUNTY COUNCIL
The event also included three moving testimonials from people enrolled in the NIH Natural History and Pathogenesis of Systemic Lupus Erythematosus study, one of whom began in the protocol 40 years ago when she was just 16 and newly diagnosed with lupus. NIH’s research had a profound effect on her quality of life.
“If participating in an NIH study can save a life…it’s worth it, because that one life may be your own,” she said.
Another described how she was recruited as a Hispanic female at a time when NIH was trying to improve diversity and representation in the lupus clinical trial. She remarked on the whole-person approach taken by the NIH, saying “when you hear the word study, you think that you are going to be a lab rat, and that has not been my experience.” She continued by saying that the NIH team showed “that they cared more about me as a person than the study.”
Another patient, who is also a patient advocate, echoed the importance of participation, noting she was able to “learn, inform and educate” through her experience.
May was Lupus Awareness Month, which the Montgomery County Council marked with a proclamation recognizing NIH’s sustained and groundbreaking work on the disorder with a presentation by Councilman Will Jawando.
Jawando spoke about his wife’s diagnosis with lupus and how women are much more likely to be diagnosed than men.
Describing disparities in lupus diagnoses, Jawando noted that “Black and Latinx women are more likely to have lupus at a younger age and are more likely to have severe symptoms.”
Addressing such disparities is an important reason that NIH strives to ensure diversity and representation in all of its clinical trials, the event illustrated.
Learn more and support NIH’s lupus research efforts by visiting: https://go.nih.gov/VKADg2q.
Watch the D.C. Lupus Consortium videocast at https://videocast.nih.gov/watch=54866. See the Montgomery County Proclamation at https://bit.ly/3SkOKz4.
