NIH to Prioritize Human-Based Research Technologies
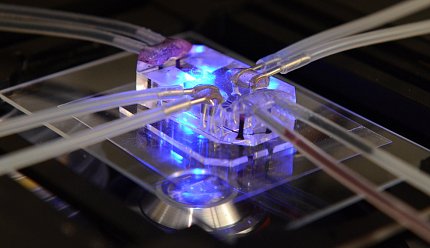
Photo: WYSS INSTITUTE FOR BIOLOGICALLY INSPIRED ENGINEERING, HARVARD
NIH is adopting a new initiative to expand innovative, human-based science while reducing animal use in research. Developing and using cutting-edge alternative non-animal research models aligns with the U.S. Food and Drug Administration’s (FDA) recent initiative to reduce testing in animals.
While traditional animal models continue to be vital to advancing scientific knowledge, using emerging technologies can expand the toolbox for researchers to answer previously difficult or unanswerable biomedical research questions.
Some animal models do not translate well to human diseases, limiting researchers’ abilities to develop effective interventions. New and emerging technologies have begun to allow researchers to study health and disease using human information, making them an alternative avenue to yield replicable, translatable and efficient results either alone or in combination with animal models.
These technologies include:
- Organoids, tissue chips and other in vitro systems that allow scientists to model human disease and capture human variability and patient-specific characteristics
- Computational models which simulate complex biological human systems, disease pathways and drug interactions
- Real-world data that allow scientists to study health outcomes in humans at community and population levels
To integrate innovative human-based science, NIH intends to establish the Office of Research Innovation, Validation and Application (ORIVA) within NIH’s Office of the Director. The new office will coordinate NIH-wide efforts to develop, validate and scale the use of non-animal approaches across the agency’s biomedical research portfolio and serve as a hub for interagency coordination and regulatory translation for public health protection.
ORIVA will expand funding and training in non-animal approaches and awareness of their value in translational success. New funding opportunities will include evaluation criteria that assess methods based on their suitability for the research question, context of use, translatability and human relevance. Infrastructure for non-animal approaches will also be expanded to make these methods more accessible to researchers.
In addition, grant review staff will participate in mitigation training to address any possible bias toward animal studies and integrate experts on alternative methods into study sections. NIH will also publish annual reports on research spending to measure progress.
