Repurposed Drug May Delay Menopause, Slow Ovarian Aging
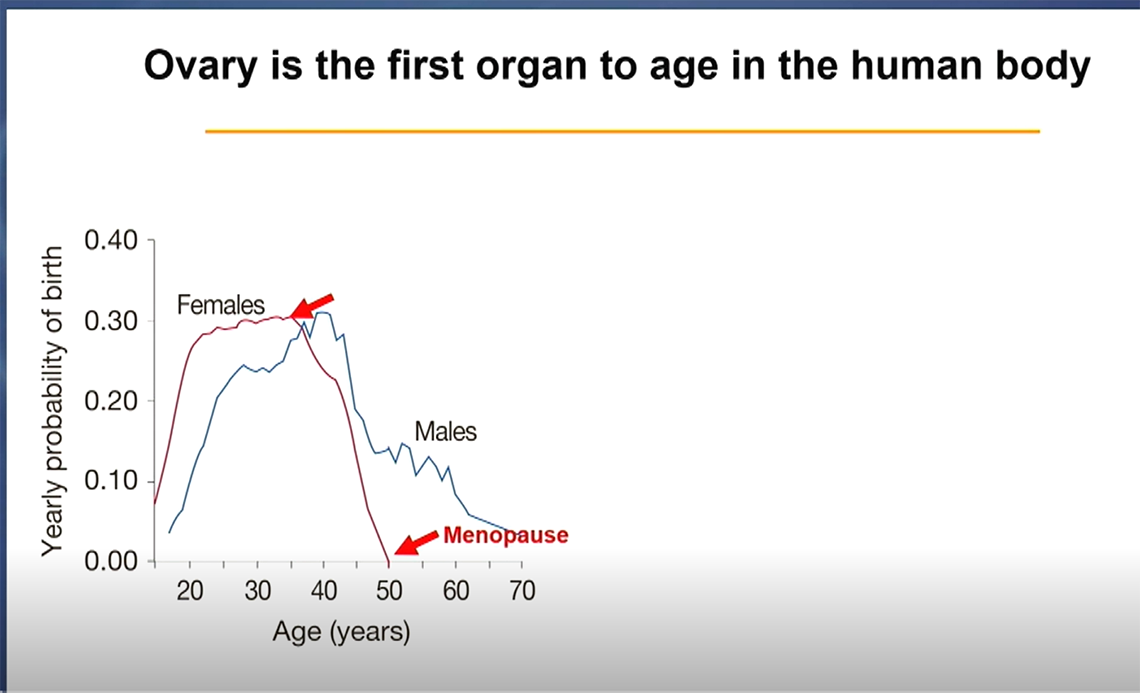
What is the first organ in the human body to age? The ovary is the first and fastest organ to undergo aging.

Ovarian aging, which culminates in menopause—the point in a woman’s life where her periods have stopped permanently and she can no longer become pregnant—has profound consequences not only for fertility, but also for overall health.
Dr. Yousin Suh, director of reproductive aging at Columbia University, discussed the biological mechanisms of ovarian aging at a recent NIH Wednesday Afternoon Lecture (WALS) in Lipsett Amphitheater. She is investigating how and why it occurs, and why it progresses so rapidly. Suh believes that understanding these processes may ultimately help delay ovarian aging and, in doing so, improve women’s health and longevity.
“Aging is the single greatest risk factor for disease,” she said, and menopause “accelerates biological aging.”
Menopause 101
Women in the U.S. tend to reach menopause between 45 and 55 years old, but ovarian function begins to decline in the mid-30s. This occurs as the ovarian follicles—often called the “functional unit” of the ovary because they house immature eggs and develop one each menstrual cycle—are gradually depleted. When a woman reaches menopause, the ovarian follicles are nearly exhausted, heralding the end of fertility.
Another major aspect of menopause is a drop in circulating estrogen levels— a hormone made by ovarian follicles that helps regulate many systems in the body. That’s why menopause can bring a wide range of symptoms. Women who undergo menopause later tend to live longer than those who experience early menopause. Interestingly, brothers of women who enter menopause later also tend to live longer, suggesting that shared genetic factors may influence both reproductive and overall aging.
Suh believes the source of these variations lies in genetics. “Aging is not simple wear and tear, but governed and controlled by underlying biology,” she explained.
Learning from Genetics
As a geroscientist, a researcher who studies how aging drives disease, Suh believes that delaying ovarian aging could help women live longer, healthier lives—a concept known as geroprotection.
To better understand why menopause timing varies so widely—from the mid-30s to the late 50s—Suh’s lab turned to human genetics. Drawing on published genome-wide association study (GWAS) data from hundreds of thousands of women, her team focused on identifying genetic variants that may play a causal role in determining the pace of ovarian aging.
While previous GWAS had uncovered hundreds of associated loci (the specific location of a gene on a chromosome), the majority lie in non-coding regions, making it difficult to pinpoint their biological impact. To bridge this gap, Suh’s team created a single-nucleus multiomics atlas of the human ovary, combining gene expression and chromatin accessibility data from young and reproductively old donors (in their 20s and early 50s, respectively).
By integrating this atlas with GWAS findings, they identified more than 100 likely causal regulatory variants and their target genes. One variant associated with later menopause, rs3741605, reduces expression of the HELB gene—an inhibitor of an accurate DNA repair pathway known as homologous recombination—suggesting a mechanism by which genetic differences might delay ovarian aging.
Importantly, the influence of the HELB variant extends beyond the ovary. “Ovarian aging-associated variants may potentially have impacts across all human tissue types,” Suh explained. This includes men, reinforcing the idea that reproductive aging genetics are not confined to the ovary alone.
Within the ovary, these variants exert broad influence.
“Menopause timing-related variants have a global impact on gene regulatory networks across the different cell types of the ovary,” Suh noted. “This integrative approach allowed us to connect genetic risk factors for menopause timing with molecular changes in ovarian cells. It helps explain the enormous variation we see across women and may ultimately point to targets for preserving reproductive health.”
Hallmarks of Aging
When Suh’s team analyzed gene expression in ovarian cells from young and older women, they identified more than 3,000 genes whose activity changed with age. What stood out was the remarkable coordination of these changes: nearly all ovarian cell types showed age-related shifts in the same direction and magnitude—an unusually unified pattern not typically seen in other human tissues during aging.
“This coordinated cellular change is a striking feature of ovarian aging,” Suh said.
Further analysis revealed that these age-sensitive genes are enriched for several well-known biological pathways collectively known as the “hallmarks of aging”—processes such as genomic instability, impaired mitochondria function, and altered nutrient sensing. These hallmarks have been widely studied as potential targets for gerotherapeutics, drugs that aim to delay or reverse biological aging and extend healthspan in preclinical models.
Among the pathways Suh’s team identified, mTOR signaling stood out for its consistent activation across multiple ovarian cell types. The mTOR pathway helps cells regulate growth and energy use, and its dysregulation is a known driver of aging.
“mTOR is activated across multiple ovarian cell types and represents a unique mechanism in reproductive aging,” she said.
Previous research has shown that rapamycin, a drug that inhibits mTOR, is one of the most effective geroprotectors in animal models. Suh’s findings suggest that the human ovary itself may be a direct target for rapamycin’s geroprotective effects.
VIBRANT Study
Encouraged by these discoveries and preclinical data, Suh launched a first-in-human clinical trial. In this pilot study called VIBRANT—Validating Benefits of Rapamycin for Reproductive Aging Treatment—50 healthy reproductive-age women were enrolled. Half received rapamycin weekly for three months. All participants were then monitored for nine months.
“We received over 200 emails from women eager to enroll when the study went live,” Suh recalled.
This first VIBRANT trial served as a proof-of-concept: it aimed to test the safety and feasibility of short-term rapamycin use and its potential effects on ovarian function. The drug was well tolerated, with no significant adverse events, and some participants reported unexpected benefits like improved memory and overall well-being.
One possible mechanism is that rapamycin reduces the number of follicles activated each month—effectively preserving the ovarian reserve and potentially delaying reproductive aging. Building on this foundation, the next phase—VIBRANT II—will expand to a multi-center trial enrolling about 200 women.
Big Picture
Studying ovarian aging could offer broader lessons for the field of aging biology. “The ovary ages earlier and faster than any other organ,” she said. “The process is so organized it looks like it’s prompted by a signal.” This makes the ovary a compelling model for aging studies—compact, time-compressed, and genetically tractable.
Suh concluded, “What we learn from female-based aging research could benefit everyone—including men.”
