‘A Necklace of Breakthroughs’
NIH Lays Groundwork for Advances in Brain Imaging

Photo: Dana Talesnik
Imaging techniques have come a long way. Their increasing sensitivity continues to push the boundaries of what’s possible to visualize in disease research, diagnostics and patient care.
It’s been nearly two years since Massachusetts General Hospital (MGH) rolled out its Connectome 2—an ultra-high-resolution brain imaging system. Data is now starting to emerge that reveals the power and potential of this technology. The scanner lets scientists and clinicians study the architecture of the human brain where they can see microscopic brain structures with incredible precision.
It’s not widely known that developing this scanner involved collaboration with NIH researchers. Underpinning this revolutionary technical feat was decades of research conducted right here on the NIH grounds.
“The motivation for building the scanner, the promise of what it could do, was based on 25 years of microstructure imaging research conducted at NIH,” said Dr. Peter Basser, senior investigator who now heads the Section on Quantitative Imaging and Tissue Sciences in the Division of Intramural Research in NIH’s Eunice Kennedy Shriver National Institute on Child Health and Human Development (NICHD).
The research, much of it performed in Basser’s lab, enables measurements of quantities like cell size, shape and orientation, among others. Initially, they could measure these features in fixed tissue specimens or in small animals, but clinical magnetic resonance imaging (MRI) scanners were not powerful enough to make these measurements.
“Now, the technology is at the point where we can make those measurements in people.”
Moving the dial
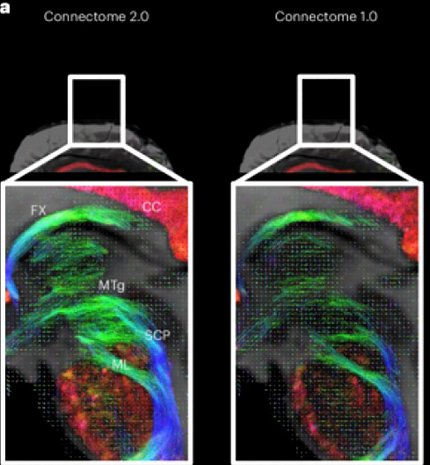
Photo: Dr. Chiara Maffei
The latest imaging analysis tools developed by Basser and colleagues—incorporated into the Connectome 2—can produce quantitative imaging biomarkers sensitive to different biological features, structures and processes.
The technology offers a radiologist more “stains” and “contrasts” to be able to see distinct features; it allows an oncologist to more easily distinguish cancer from normal tissue; it allows neuroscientists to more clearly see connections in the brain. Beyond the brain, there are many other possible applications, from cardiac muscle imaging to soft tissue studies.
In the early years, Basser recounted, other scientists doubted the future utility of microstructure imaging research. Also, it took time for the imaging technology to catch up to the modeling approaches they now use. But it turned out, with the right equipment, this technology allows researchers to see structures in the nervous system and other parts of the body that they couldn’t see before.
“That’s what NIH’s Intramural Research Program [IRP] is all about,” Basser said. “We take risks. We can try things here that are not guaranteed to work, but if they do, they can ‘move the dial.’”
A natural marriage
For the past few decades, NIH de-risked the basic imaging science and moved the microstructure imaging research to the point where it could be clinically translated. Enter MGH Martinos Center—eager to migrate the software and methodologies of microstructure imaging for use in the clinic. MGH had the technology and connections with manufacturers to develop the Connectome 2 and sought to incorporate the MRI methods that NIH developed.
MGH invited NIH to collaborate. Together, they applied for an NIH BRAIN Initiative® grant to build a scanner that ultimately could be used with patients in approved clinical trials. It was a truly symbiotic relationship.
“It’s a perfect example of what the NIH IRP does well—behind-the-scenes research that underpins important technical and clinical developments,” Basser said. “It was a natural marriage between our group and theirs—between intramural and extramural.”
Basser added, “I wish more people recognized that when they receive a therapy that makes them better, or when they take a diagnostic test that helps doctors better understand what condition they may have, it didn’t just happen by itself. There was an infrastructure—an entire ecosystem producing these technologies.”
Precursors to the Connectome

Photo: Dana Talesnik
Before starting his own bioengineering lab at NIH, Dr. Carlo Pierpaoli worked with Basser to develop diffusion tensor imaging (DTI), an MRI technique that tracks the direction of water molecules to map the brain’s white matter. The Connectome 2 is a next-generation system that can identify features and structures that DTI can’t detect.
But in those earlier days, “Carlo was probably the one most responsible for translating DTI from bench to bedside, from our early experiments with fixed tissue to imaging in the clinic,” said Basser.
Other MRI advances developed in Basser’s lab include a tracking method that creates a “wiring diagram” of white matter connections in the brain; ways to identify brain cortical folds, areas and layers; and imaging approaches to acquire key neuro-anatomical and histological information.
A foundation built on investments
The development of MRI technology dates back to the 1920s, when physicists Stern and Gerlach identified magnetic properties of nuclear spins. Every decade since, scientific breakthroughs in nuclear processes led to the development of MRI.
“Things we’re doing now are based on investments made literally 100 years ago and developments that resulted in numerous Nobel Prizes along the way,” Basser said.
Along the way, basic science becomes the new technology.
“The connection is very obvious in the MRI field,” he said, “because you can easily see how one breakthrough led to another and another, bringing us to where we are now. It’s basically a whole necklace of breakthroughs.”
Steadfast research, vast potential
Basser has been an investigator at NIH for nearly 40 years. He’s grateful for the resources and support of NIH’s IRP.
“Given my profile as a scientist-inventor, the NIH IRP was a good fit for me because I could try things here—DTI for example, that took 10 years to elicit interest in the radiology field—and advance them to the point where companies considered licensing the technology from NIH.”
That’s one feature that makes NIH so special. There’s a long lead time in instrumentation development—design, testing, licensing and regulatory approval. “It would’ve been difficult to keep a lab [anywhere else] funded before these methods became widely used.”
The contributions of Basser and collaborators and the potential of these ever-sharpening technologies are vast. Basser is proud of the steadfast research that enabled these capabilities with the promise to transform patient care.
“Our scanning techniques are used everywhere,” he said. They’re improving diagnosis and outcomes. “It’s beholden to us [at NIH] to advocate for ourselves and tell the public how we’re improving their lives. Hopefully, we are doing this better now!”
