Therapy for Rare Bone Disorder Shows Promise
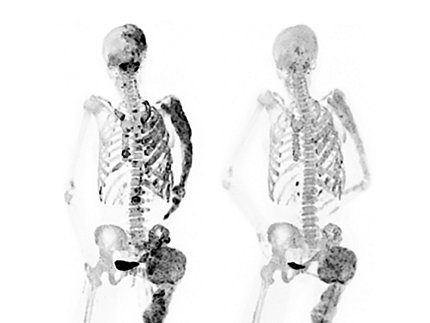
Photo: Alison Boyce/NIDCR
A clinical trial at NIH found that a medication, denosumab, significantly reduced abnormal bone turnover in adults with fibrous dysplasia, a rare disease marked by weak and misshapen bones. Bone turnover, a process in which old bone is continuously replaced with new bone, is unusually accelerated in fibrous dysplasia and contributes to bone abnormalities.
The study of eight participants was carried out by researchers from NIDCR and the Clinical Center. Results, which showed that denosumab may improve patients’ quality of life by enabling healthy bone formation, were published as a correspondence report in the New England Journal of Medicine.
Fibrous dysplasia stems from gene mutations that cause scar-like (fibrous) tissue to replace healthy bone starting in early childhood. These fibrous lesions—marked by accelerated bone turnover—weaken bones, leading to bone deformities, fractures, physical disabilities and pain. In some cases, the lesions can press up against organs and nerves, impairing functions like vision and breathing.
“Surgery is still the standard treatment for fractures and deformities caused by fibrous dysplasia,” said senior author Dr. Alison Boyce, a clinical investigator at NIDCR. “Denosumab is the first medication that appears to affect how fibrous dysplasia lesions behave and improves patients’ disease outcomes.”
