‘Dare to Rethink’
Holland Offers Inspiration, Wisdom at CC Grand Rounds

In their quest for solutions, most scientists know failure all too well.
“Don’t be afraid of the failures; you will learn more from them than you do the successes,” said Dr. Steven Holland, who shared lessons and advice with incoming fellows at a recent Clinical Center Grand Rounds.
In a talk about his own research journey, Holland also shared aspects of what makes the Clinical Center (CC) unique and why he believes some researchers stay at NIH for so long. Holland, an NIH distinguished investigator and director of intramural research at the National Institute of Allergy and Infectious Diseases (NIAID), has worked at NIH for 34 years and counting.
The CC’s relatively stable resources enable researchers to focus on complex, enduring problems, Holland told the new arrivals. Further facilitating research, the CC has fully integrated labs and services.
“We are committed at every fascicle of the being of the organization to making sure the integration of patient care and research is complete and constant,” he said.
Holland attributed his career trajectory to former CC Director and former CC Scientific Officer Dr. John Gallin, who retired this year after 50 years at NIH. Gallin’s initial advice was to go study neutrophils—more on those later. That advice led Holland to study rare immunodeficiencies. His successes included contributing to understanding the cause of Job’s syndrome in 2008 and DOCK8 deficiency two years later.
Why Study Rare Immunodeficiencies?

“We study the rare things to understand the common things,” Holland said. Everyone is exposed to microbes. “It’s precisely the fact that those people who got sick didn’t control it that tells you they must be sick. What we’re really studying are common infections that are not well-controlled.”
There are three causes, he explained: bad microbes, bad exposures or host problems. “Our assumption is no matter which of these it is, we can figure it out.”
Upon joining Gallin’s lab in 1992, Holland recounted a call from a pediatrician about a 5-year-old boy who was critically ill, on intravenous nutrition, with M. avium complex (MAC) oozing from his blood, bowel and sinuses. The boy had two maternal uncles with the same condition. The pediatrician suspected chronic granulomatous disease (CGD).
Holland wasn’t sure about the pediatrician’s hypothesis. Disseminated MAC usually does not occur in CGD. But all signs pointed to something genetic and Holland invited the patient and his family to NIH for evaluation.
“Patients are reluctant to simply hear why they’re so sick,” Holland said. “They want to hear how you’re going to make them better.”
MAC is in the family of mycobacteria, of which there now are more than 150 known species, explained Holland. What is now Mycobacterium tuberculosis was first identified in 1882 as the cause of tuberculosis (TB). Non-TB forms of mycobacteria are plentiful in the environment—in water, air and soil—and are therefore unavoidable.
When the young patient arrived at NIH, Holland’s team considered treatment options. “That really is why we have a hospital attached to a laboratory,” said Holland. “What we have that’s unique is putting those two together.”

The team treated the child with interferon-gamma, then recently approved for treatment of a different disease. Previous patients with refractory mycobacterial infections had not responded as Holland had hoped but in this case it worked. The little boy—whose growth had stymied over that past year—got better. He went off of IV therapy and nutrition, began to grow and went on to have a normal life. The treatment would succeed in additional patients as well.
But soon after, Holland received reports of patients with severe MAC not responding to interferon-gamma therapy. And thus began a study of receptors and pathways to find alternative treatments.
Most disseminated mycobacterial diseases present in childhood, but Holland started to get referrals of adults who were not responding to interferon-gamma. One patient, he recounted, was a 60-year-old woman from Vietnam who had lesions on her chest that were teeming with mycobacteria. Despite treatment, she had persistent disease.
“The problem here is: this looks like a genetic disease, it acts like a genetic disease, but it’s coming on later in life,” he said. “So we have to be thinking a little bit further outside the box.”
Further study into adult-onset infection showed that some patients didn’t respond to interferon-gamma treatment because they had autoantibodies against interferon-gamma. Holland is still studying this population and alternate treatments.
He underscored a lesson for the trainees: Not every case is best thought through as an outpatient. Admit patients to the hospital. “We have a hospital that can do better and can do it [for free], without regard for insurance,” he said.
Talk to doctors and patients, he advised. Be skeptical. “Embrace the unexpected because that’s where the novelty is,” he said. “That’s what I hope will keep you up at night and keep you interested in doing more.”
Sticky Situation: Neutrophils
Gallin’s advice, decades ago, to study neutrophils began a lifelong journey for Holland into immune disorders. Neutrophils are a type of white blood cell with a tough job. They are required for remodeling tissue, such as forming scabs.
“They have to roll along the endothelium where they’re constantly waiting for someone to say, ‘dinner is being delivered,’” explained Holland.
When that dinner bell rings, neutrophils have receptors on their surface that bind to receptors on the endothelium; they then go out into the tissue to do the ingesting and killing that allow the tissue to heal.
But if neutrophils don’t have the right receptors, they can’t stick and then can’t travel to where they’re needed. An example is leukocyte adhesion deficiency (LAD), a rare genetic disease that leads to frequent infections and loss of all teeth before adulthood.
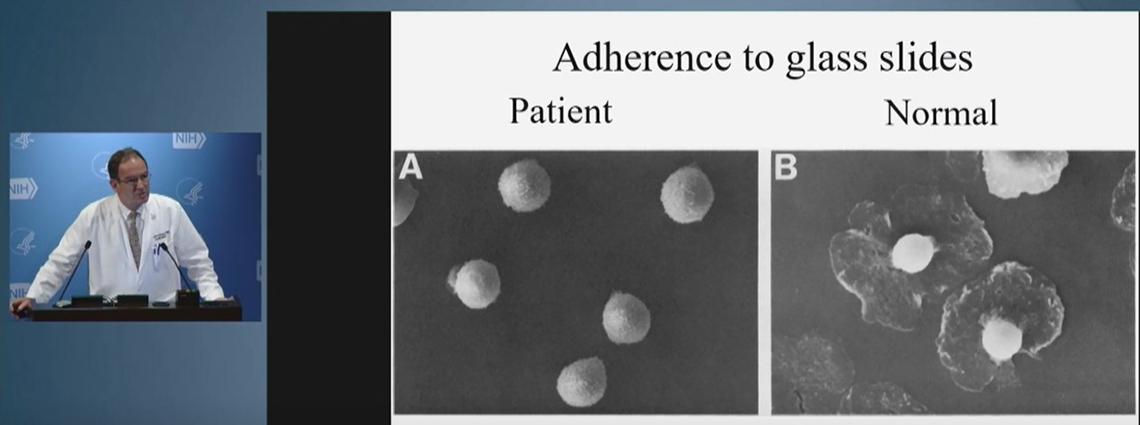
After consulting with a researcher at the National Institute of Dental and Craniofacial Research, Holland learned his hypothesis on the cause of LAD was misguided. From this now long-term collaboration, a monoclonal antibody is in clinical trials toward treating the disease.
“Dare to rethink the problem, and often,” Holland said. “Don’t let being wrong get in the way of getting it right.” Embrace mistakes. “That’s really where the opportunities for growth and development are.”
