Labs (And Collies And Terriers) Report
Ostrander Connects Canine, Human Mental Health

Children’s author Roald Dahl once said, “The greatest secrets are always hidden in the most unlikely places.”
That couldn’t be more true for National Human Genome Research Institute (NHGRI) Distinguished Investigator Dr. Elaine Ostrander, whose interest in genomics research led her to study an unassuming candidate: Canis lupus familiaris, better known as the domestic dog.
Research into dog genetics may have useful applications for human health, explained Ostrander and her fellow NHGRI scientist Dr. Philip Shaw in a recent Demystifying Medicine lecture titled “Behavioral Genetics: Man Meets Dog.”
The Demystifying Medicine series seeks to bridge the gap between advances in biology and their applications to major human diseases. This edition, host and NHGRI Scientific Director Emeritus Dr. Dan Kastner said, sought to demonstrate “how dog behavioral genetics can help us learn more about ourselves.”
What makes dogs so attractive for genetics research?
They are numerous—about 90 million in the U.S. alone—and live in and are exposed to the same environments as their humans. Dogs have shorter lifespans, so researchers can study multiple generations. And owners, veterinarians and breeders are willing to share their dogs’ data.
Ostrander’s laboratory is a citizen science project; the lab doesn’t have any of its own dogs but relies on data provided by its partners. The lab currently has about 40,000 samples.
By analyzing the genomes of individual dogs from 161 breeds, Ostrander was able to organize the breeds into a dendrogram—a branching diagram that shows the relationships of similarity among a group of entities. She used the dendrogram to illustrate the relatedness of the groups that all breeds of dog fall into—such as the herding group or terrier group—and found that they can be divided into 23 distinct clades, or groups that share a common genetic history.
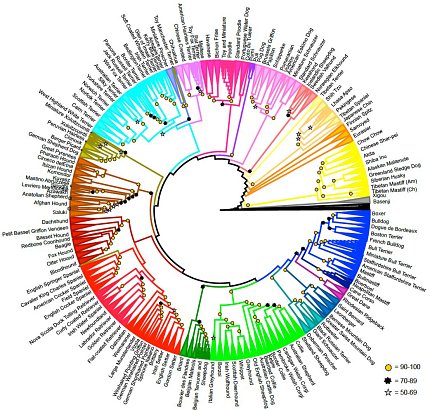
“This is what makes dog genetics so cool,” Ostrander explained. Because “dogs come with fantastic labels,” (aka breeds) researchers can use those labels to begin to break down conditions that are harder to study in humans due to our lack of clean labels. One example is locus heterogeneity, where mutations in different genes can cause the same disease or condition.
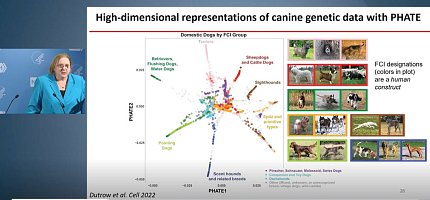
Another helpful data visualization technique Ostrander’s lab used was PHATE (potential of heat diffusion for affinity-based transition embedding), which allowed them to view dog lineages in a way that showed unlimited neighbors, rather than a bifurcate way like the original dendrogram.
Basically, Ostrander said, “it gave us more information about what led to what.”
Her next goal was to use PHATE to map dog behavior. How? By studying genes that may be associated with stereotypic breed behaviors.
Ostrander’s group decided to use the herding dog lineage which, as shown by the PHATE map, included a wide array of breeds with different herding and guarding behaviors. Ostrander focused on the true herders within the lineage.
Her lab conducted an enrichment analysis of the herders’ DNA and found that they tended to share similar genetic variants in three types of axon guidance genes, each of which helps shape brain circuitry early in development. Of those genes, many are in ephrin (EPH) pathways. Of particular interest is EPHA5, for which DNA variants are observed in three-quarters of border collies, but less than 10% of non-border collie breeds.
EPHA5 has been implicated in anxiety and maternal pup-herding in mouse models. Could the border collie’s herding drive be an augmentation of the anxiety-associated pathways that drive mouse maternal protective behaviors? That’s a question for future studies, Ostrander said, but she’s excited to investigate it.
Sometimes, though, those anxiety-associated pathways can lead to compulsive behaviors such as pacing or spinning.
“Preliminary genetic analyses have indicated genes that are implicated or associated with human psychiatric conditions [such as autism spectrum disorder, obsessive-compulsive disorder, schizophrenia and attention-deficit/hyperactivity disorder],” explained Ostrander.
Dog breeds that excel in agility competitions are more likely to have variants of a gene called ROBO1.
Interestingly, in humans, ROBO1 is associated with developmental dyslexia.
Human children who are affected may learn more effectively through images and diagrams than with written or spoken instruction, because their brains are better at processing visual information.
ROBO1 may help dogs excel at agility by helping them identify and learn environmental information, enabling them to speedily navigate obstacle courses.
These parallels between dogs and humans hold potential for future study, concluded Ostrander, and ultimately may lead to better treatments for both species.
The archived lecture can be viewed at https://videocast.nih.gov/watch=54037.
