Healthy Aging
NIA Researchers Introduce the Healthspan

Can we live to 120? And do we want to?
Researchers from NIH’s National Institute on Aging (NIA) pondered this topic in a recent Demystifying Medicine lecture. Dr. Luigi Ferrucci and Dr. Payel Sen approached these questions from two different angles: epidemiology for Ferrucci, and experimental biology for Sen.
Ferrucci, the scientific director at NIA, is a geriatrician and epidemiologist who studies the causal pathways leading to progressive physical and cognitive decline in older people. He contends that, rather than extending the lifespan, we should aim to increase the “healthspan,” or the number of years lived in good health.
“As the global population ages, we need to change our perspective on what we think about health and human care,” Ferrucci said. In his days as a practicing gerontologist, he recognized that reducing time spent in disability and frailty was often more important to his patients than increasing overall life expectancy.
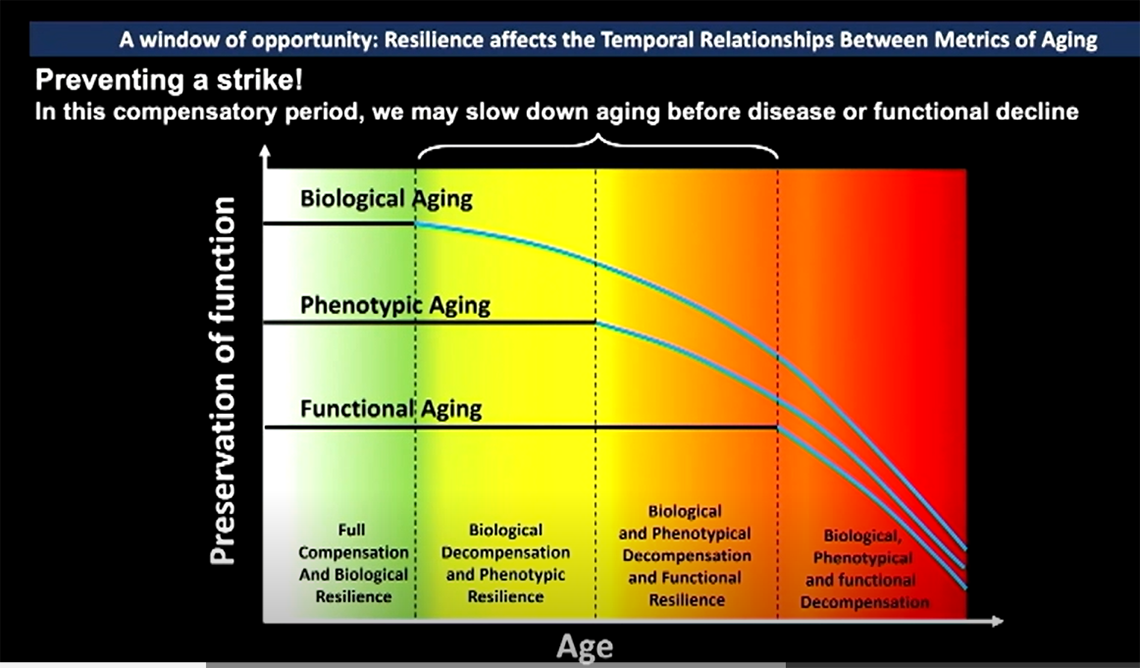
Aging is the strongest risk factor for all major age-related chronic diseases—like osteoarthritis, chronic heart disease and cancer—Ferrucci said, but he believes we are missing the window of opportunity for intervention.
Health care practitioners tend to intervene at the end of a person’s disease-free life expectancy, when chronic diseases reach the clinical threshold and begin showing symptoms. This approach is expensive, he explained: the U.S. spends 97.5% of its health care funds on people with some sort of disease, but spending money on patients already experiencing chronic disease(s) does little to expand their healthspan.
But what if we could detect and treat disease accumulation earlier in life? One way to conceptualize aging, Ferrucci said, is the “accumulation of macromolecule and cellular damage that eventually becomes pathology.” By this, he means the buildup and deposition of abnormal proteins or other substances within cells, tissues or organs, which can lead to various diseases. If we could counter or slow this accumulation, then we could potentially reduce susceptibility to chronic diseases in older people.
The aging process tends to damage randomly different cellular structures. For example, DNA damage that remains unrepaired leads to mutations or transcription errors, and the burden of damage accumulated reaches a critical threshold that may lead to adverse cellular responses such as neoplastic transformation, cellular senescence or apoptosis. The accumulation of DNA damage also affects its functionality, causing degenerative diseases.
Because of the resiliency of our bodies, Ferrucci said, we can afford some biological damage without showing any outward (or “phenotypic”) signs. Phenotypic aging is the third stage, and manifests as noticeable changes such as low muscle strength or changes in visual or hearing acuity. In the final stage, the aging process has progressed to functional challenges, during which individuals may experience problems with memory or mobility, among other impairments.
Not everyone ages at the same rate, and inherited and external factors also influence the aging process. “Investments in early life, particularly in utero, may help postpone age-related morbidity and mortality and extend the healthy lifespan,” Ferrucci noted.
Sen’s portion of the lecture focused on the effects of epigenetics on aging. Epigenetics is the study of how environmental factors can alter gene expression without changing the underlying DNA sequence. To highlight the difference, she referenced a study comparing the lifespans of twins.
“Genetics can only explain 25% of the lifespan variation in twins,” she said. “The rest is epigenetics.”
One way to learn more about aging is by studying organisms and species that are different from us. In comparative biology, which involves studying different organisms to understand the patterns of life at all levels, researchers have found that maximum lifespan is positively correlated to body mass. Larger species such as whales and elephants have the longest lifespans, while rodents trend in the opposite direction for both size and lifespan.
Humans are one exception, Sen noted, being one of the longest-lived mammals despite being much smaller than other mammals with similar lifespans. “That may be a sign that we’ve already pushed the limits of our longevity,” she said.
Another famous exception is the naked mole rat, which can live up to 30 years. By studying them, researchers hope to gain insights into how these rodents acquired their longevity.

Aging and longevity studies on numerous categories of model organisms have revealed “repetitive molecular pathways” that emerge during aging. These mechanisms when they become dysfunctional are known as hallmarks of aging. Researchers have identified 12 hallmarks, which fall into three distinct categories: primary, antagonistic and integrative.
Primary hallmarks are the earliest, most causal changes—such as epigenetic alterations, Sen’s area of research. Antagonistic hallmarks are cellular responses to primary changes, and integrative hallmarks are responses seen on a systemic level.
Cellular senescence—an antagonistic hallmark—creates “zombie cells,” which release pro-inflammatory molecules and are also harder to destroy. Researchers created a molecule that initiated destruction of senescent cells in a mouse model, which improved both healthspan and lifespan for the animals.
Chronic inflammation occurs at multiple levels throughout the human body. As an integrative hallmark, chronic inflammation can increase with age and even drive aging in other organs.
Are age-related molecular changes reversible? Researchers have identified a few promising interventions in animal models, but these are preliminary and will also be harder to translate into use for humans.
In the meantime, Ferrucci and Sen agree that healthy lifestyle choices such as eating well, prioritizing exercise and sleep, maintaining social ties and continuing to learn new things are the best ways to set yourself up for healthy aging.
“A healthy lifestyle is the key in the lock of creating successful, long-lived societies,” Ferrucci said.
To view an archived version of the lecture, please visit https://go.nih.gov/0mJjkVz.
