More Rapid Relief
Zarate Advances New Options to Treat Severe Depression

This story is part of our ongoing series highlighting NIH makers.
When seeing patients over the years, Dr. Carlos Zarate noticed troubling trends. Existing treatments for clinical depression were often inadequate. His patients were suffering. Not only did they need more immediate relief, but some of them could find no relief at all.
More than 280 million people around the world suffer from depression, one-third of whom do not respond at all to existing medications or don’t experience a sustained response. It’s this group—the millions who have treatment-resistant depression—who inspired Zarate to seek new solutions.
Zarate, who has studied mood and anxiety disorders at the NIH for 25 years, is chief of the Experimental Therapeutics and Pathophysiology Branch at the National Institute of Mental Health (NIMH). When testing dozens of different antidepressants in clinical trials, he found that, “a good number of people did not achieve remission, meaning fewer to no symptoms,” he said. If they did respond, “it often would take weeks or months for them to have an antidepressive response.”
In many cases, it took at least six months to achieve any kind of remission. “That’s a long time to ask people to hang in there for things to get better,” he said.
Zarate’s team spent many years studying different cellular and molecular pathways and potential medications that could bring about rapid antidepressant effects. A decade ago, he homed in on ketamine, an anesthetic approved by the Food and Drug Administration (FDA) that is also used to treat pain.
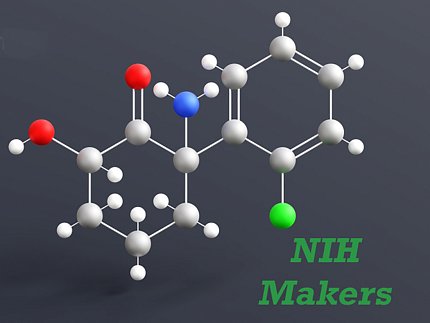
Photo: Sergei Shimanovich/Shutterstock
The drug enters a brain glutamate receptor called NMDA and blocks it. “That resulted in a rapid antidepressant response, meaning what you get in a few hours was equivalent to what takes six weeks or longer with a traditional antidepressant,” Zarate said.
What’s more, ketamine showed promising results in individuals who did not improve after trying different antidepressants or electroconvulsive therapy (ECT); many of these individuals also had previous suicide attempts.
But there was an overshadowing drawback: ketamine can be abused.
“Even though we found rapid antidepressive effects,” noted Zarate, “because of its dissociative side effects (temporary detachment from reality), there’s a potential for misuse. So, industry had many reservations about developing this drug.”
In controlled NIMH studies, Zarate’s team tested ketamine intravenously and were co-inventors of a nasal spray version. Over time, they replicated results and ultimately demonstrated that ketamine could be administered safely. Other researchers began building on their findings, leading to independent replication.
In 2018, Janssen licensed the esketamine nasal spray, called Spravato, from NIH and its Yale and Mt. Sinai collaborators. A year later, the FDA approved Spravato—when combined with an oral antidepressant—for treatment-resistant depression in adults with the stipulation that it be administered by a healthcare provider in a medical facility. In 2020, the FDA approved Spravato for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior.
In January 2025, the FDA expanded its approval of Spravato as a stand-alone therapy in adults with severe depression. Esketamine, administered under medical supervision, is now approved in more than 90 countries and used increasingly around the world.
Meanwhile, Zarate’s lab continued to seek improved treatments.
“Now we know ketamine works,” he said, “so how can we re-engineer it to better understand its mechanisms?”
A breakthrough came several years ago, after a series of studies NIMH conducted in conjunction with the National Institute on Aging (NIA), the National Center for Advancing Translational Science (NCATS) and the University of Maryland. They focused on one of ketamine’s metabolites—hydroxynorketamine (HNK)— that does not have ketamine’s dissociative side effects or misuse potential. The investigators synthesized HNK for more preclinical experiments and eventual human use.
Zarate and colleagues published results of a Phase-1 study in healthy volunteers showing that HNK is safe, and they’ve now begun a Phase-2 trial in patients with treatment-resistant depression. In these studies, investigators administer HNK intravenously, but it has the potential to be developed into an oral antidepressant that ultimately could reach many more patients.
Along the way, Zarate has faced ongoing challenges. “We had to pay close attention to ethical and moral considerations,” he noted.
One such consideration when conducting mental health research is whether the patient has the capacity to consent to an experimental therapeutic, or any treatment.
Also, at the forefront of consideration in pursuing this line of research is the misuse potential of ketamine—particularly in people with depression and among those who have had prior substance use challenges. Zarate recounted multiple meetings with interdisciplinary teams—physicians, nurses, social workers, nutritionists—to devise a plan to address patients’ needs and staff concerns when studying ketamine.
He said, “In the end, it took us quite a bit of time to get these studies up and running, but a lot of good has come out of it.”
Going forward, Zarate’s team is studying organoids derived from patients with the goal of further improving treatments.
“If we can find common changes in gene expression or changes in proteins with distinct rapid-acting antidepressants, that could offer a new target to pursue for development,” he said.
One of the most exciting parts of this journey, he added, is working with trainees and watching this program develop.
“We’ve gone from mainly a clinical program to a biological clinical translational program,” Zarate said. “We go from the clinic to the bench and back and forth to see if we can develop better insights that we should pursue.”
