NIBIB Grantee Tests Efficacy, Appeal of Flu Vaccine Patch
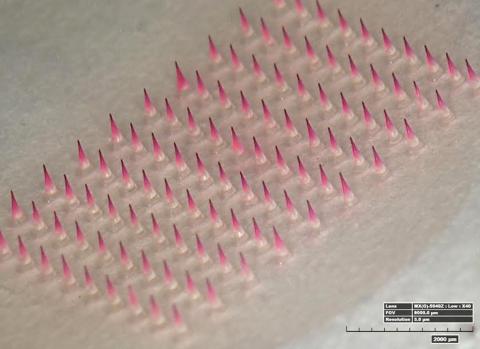
Photo: Devin McAllister/Georgia Tech
A dime-sized patch of tiny, dissolvable microneedles could be the biomedical advance that expands the reach of vaccines to remote parts of the world and overcomes fear that prevents many from getting a flu shot each year. Dr. Mark Prausnitz, Regents professor and J. Erskine Love chair in chemical and biomolecular engineering, Georgia Institute of Technology, presented the microneedle technology and results from his research leading up to a phase 1 clinical trial during a recent seminar at the National Institute of Biomedical Imaging and Bioengineering.
Prausnitz receives funding from NIBIB, including a special Quantum grant, a mechanism for profound advances in biomedical engineering technologies that address major diseases or national public health problems.
The Food and Drug Administration granted investigational new drug status for the vaccine and novel administration method, allowing Prausnitz and collaborators at Emory University School of Medicine to conduct a study that started last fall using the microneedle patch. They assessed the safety of the engineered device, how the body’s immune system responded to the vaccine delivered through a patch and participants’ opinions about using the patch. Researchers from the international health organization PATH evaluated policy implications around a vaccine that just about anyone could deliver.

Photo: NIBIB
The vaccine patch consists of 100 solid, water-soluble needles that are just long enough to penetrate the skin. Adhesive helps grip the skin during the administration of the vaccine, which is encapsulated in the needles and is released as the needle tips dissolve. In the clinical trial, the researchers allowed 20 minutes for the needles to dissolve in the skin, but are aiming for 5-minute stick times in the future. The patch is peeled away and discarded like a used bandage strip.
The patient or a minimally trained person can administer the microneedle vaccine patch, applied at the wrist with thumb pressure. In order to have minimally trained personnel apply the vaccine in the trial, bioengineers designed a simple feedback mechanism to indicate sufficient pressure. The feedback is provided in the form of a click that can be felt and heard.
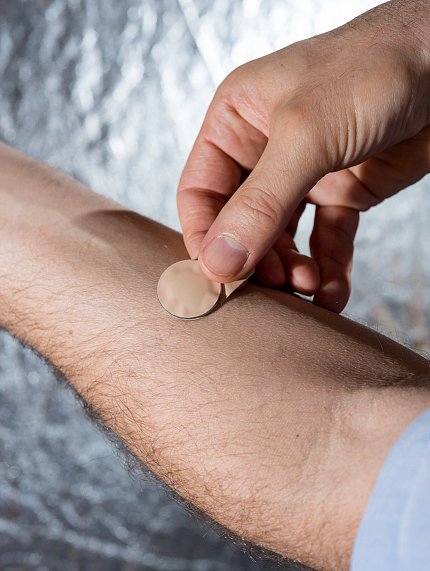
Photo: Rob Felt/Georgia Tech
“We think if we build it they will come,” Prausnitz said. “We wanted to find out if it’s true that people would like to get their flu vaccine using a microneedle patch.”
Nearly 100 adult participants were enrolled in the study to assess the appeal of flu vaccination using microneedle patches. In a figure that aligns closely with the wider U.S. population, 46 percent of the study participants had intentions to get a flu vaccine. Each got to experience administration of a patch as well as a syringe injection, neither of which contained vaccine. Then researchers offered four choices for future vaccination: take a patch home and self-administer the vaccine, have a health practitioner supervise the patch vaccination, have the health practitioner administer the patch, or the standard injection. The large majority of volunteers wanted to use the patch and to administer the vaccine themselves. Some of the participants who hadn’t planned to get a flu vaccine had a change of heart; 35 percent of them opted to be vaccinated once given the choice of the microneedle patch.

Photo: NIBIB
Prausnitz says that in addition to patients preferring microneedle patches, these skin patches may enable flu vaccine to be more effective because it interacts with the skin, rather than the muscle layer beneath the skin. “The skin is an immune surveillance organ,” he said. “It’s our interface with the outside world, so it’s very well equipped to detect a pathogen and mount an immune response against it.”
The prospective vaccine technology offers economic and manufacturing advantages too, according to Prausnitz. The manufacturing cost for the patch is expected to be competitive with prefilled syringe costs. The patch, however, can dramatically reduce the cost of vaccination, since self-administration can eliminate the need to have health workers oversee the process. It can be easily packaged for transportation, requires no refrigeration and is stable. The researchers saw no degradation of the vaccine in microneedle patches after a year stored at room temperature.
Data from the clinical trial of flu vaccination using microneedle patches, which is the culmination of Prausnitz’ Quantum grant, are currently being analyzed. Publication in a scientific journal is expected soon.
