What the Body Remembers
Virologist Delves into Covid Immunity

An ongoing quandary in the Covid-19 pandemic is immune response. How well and for how long does the body’s immune system remember a previous infection or vaccination to help ward off, or minimize symptoms during, a future infection?
Such questions continue to drive the research of Dr. Shane Crotty, virologist and professor at the La Jolla Institute for Immunology (LJI) at UCSD School of Medicine. He discussed adaptive immunity at a recent NIAID Covid-19 scientific interest group virtual seminar.
Crotty’s lab has led groundbreaking research on viral immune memory. In fact, his team’s research on Covid-19, in collaboration with Dr. Alessandro Sette at LJI, helped inform vaccine efforts worldwide.
In the early months of Covid-19, when so much was unknown, Crotty’s lab found that people with average, non-hospitalized Covid-19 infections made antibodies to the spike protein. In a co-authored paper in Cell, his team confirmed that after someone contracts SARS-CoV-2, the virus that causes Covid-19, the body activates all three components of adaptive immunity—CD4 “helper” T cells, CD8 “killer” or suppressor T cells and neutralizing antibodies.
These and related studies signaled to vaccine manufacturers that they were on target in developing spike-only vaccines.
Remembering Covid
Immune memory is tough to predict and requires waiting for a set endpoint. Time was of the essence though in trying to determine whether past infection protected against reinfection.
Following a cohort, Crotty’s lab found that most participants still had antibodies in the blood at eight months post-infection.
Antibody titers “are relatively low in infected people, but something like 90 to 98% of people do become antibody positive,” said Crotty. “Those titers drop over a relatively short timeframe but by six months, they’re relatively stable.”
Data in the literature supports that a year to two years out, these infection-induced antibody titers are stably maintained.
“The CD4 responses to this virus are quite robust,” Crotty said. “Almost 100% of people were positive initially and still 90% of people were positive at six to eight months [with data] suggesting that both CD4 and CD8 both would be long-lasting.”
That 2020 study followed 188 people, then by far the largest study of its kind involving any acute infection. Most surprising, he said, was that people had more memory B cells—the cells that create antibodies—several months post-infection than at one month post-infection.
Results pointed to substantial immune memory following Covid-19 infection, enough to “protect at least from serious SARS-CoV-2 infection for years into the future,” he said. “This was before the existence of variants.”
Four Vaccines Go Head-to-Head
Subsequent studies showed that Covid-19 infection itself did not elicit high and durable neutralizing antibodies, given the emergence of new strains. Meanwhile, growing evidence pointed to T cells helping to protect against severe disease.
Therefore, “it’s reasonable to consider that hospitalization-level Covid-19 is prevented by any decent combination of antibodies, memory B cells, CD4s and CD8s,” said Crotty.
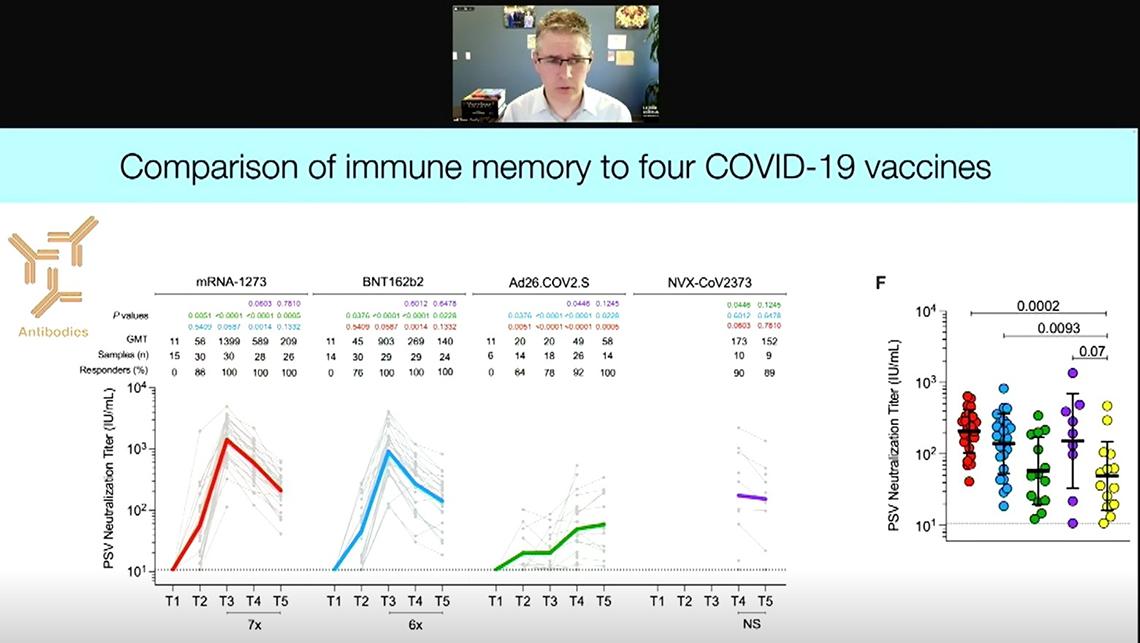
Earlier this year, Crotty’s lab published a study that compared immune memory generated by four Covid-19 vaccines: the two mRNA ones—Moderna and Pfizer-BioNTech; the viral vector Johnson & Johnson (J&J); and the protein-based Novavax.
“The RNA vaccines are fantastic at eliciting high neutralizing antibody titers quickly and consistently across individuals, but with a dramatic decay over a six-month period,” said Crotty. And they continue to decay for 10 months after a two-dose regimen.
Six months after vaccination, Novavax showed antibody titers comparable to the RNA vaccines, which were significantly higher than people get after infection or from a single dose of J&J. Interestingly, though, the J&J vaccine elicited low antibody titers one month after vaccination, those titers increased over time and that cohort had higher on average antibodies six months post-vaccination.
“This is literally the opposite trend of what you get with RNA vaccines,” noted Crotty, “indicating that these vaccine platforms are engaging different immunological mechanisms of action.”
The RNA vaccines and Novavax elicited good CD4 T cell responses up to six months. All four vaccines generated B cell memory, though the RNA vaccines performed best. Interestingly, memory B cells continued to increase three and six months out, as opposed to the antibody titers, which declined over time—a trend also seen post-infection.
Notably, Crotty’s team discovered a new type of helper cell—Tfh, a subset of CD4 T cells that are critical in the body’s fight against Covid-19. It turns out, the vaccines all generate Tfh cells. “They’re certainly required for all of the Omicron [variant] neutralizing antibodies you currently have circulating in your blood,” said Crotty.
“I think the potency of RNA vaccines for eliciting neutralizing antibodies—and being so highly protective so quickly—really comes down to their impressive ability to prime CD4 T cell responses and drive Tfh cell differentiation,” he added.
In addition to generating Tfh cells, he said, the vaccines also generate memory Tfh cells, important for booster responses at subsequent time points.
Beyond Blood: Looking at Tissues
Testing for antibodies in the blood measures circulating memory. Does the human body have additional layers of defense?
Crotty collaborated with Dr. Donna Farber’s lab at Columbia University to test whether past infection elicited tissue-resident memory. Looking at donated tissue from four people who died from non-Covid causes and had a previous unremarkable case of Covid-19, they found all had tissue-resident memory CD4 T cells and virus-specific memory CD8 T cells in the lungs.
This study occurred before Covid vaccines were available, confirming the four people had infection-induced immunity. When people are vaccinated, they get the benefit of hybrid immunity, getting tissue-resident memory cells from previous infection as well as strong circulating memory generated by vaccination.
“For me, having been trained as a virologist, I think it’s always critical to think of protective immunity in the context of the anatomy and pathogenesis of the particular viral infection that you are trying to deal with,” said Crotty. “With SARS-CoV-2, this is clearly a virus that is very rapid at replicating in nasal and oral cavities and very rapid at causing asymptomatic to cold-like disease, and so it’s a rather difficult virus to stop quickly.
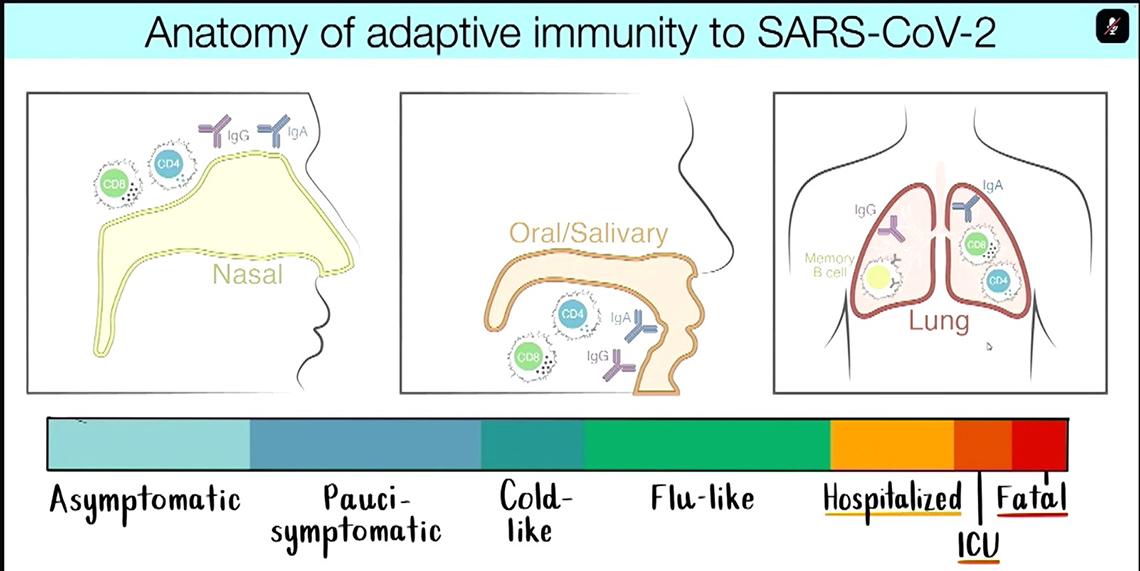
“But in terms of preventing death and hospitalizations, it’s actually a relatively slow pathogenesis, dependent on infection in the lung, [which often doesn’t occur until] two weeks post-infection or symptom onset.”
So it’s important to distinguish between protecting against infection versus serious disease, he said, and to keep in mind the different tissues and organs affected by infection.
Immune Memory from Vaccines
Crotty’s studies have shown Tfh cells start helping memory B cells early in the immune response, as early as the first few days, signaling the B cells to undergo mutation and proliferation.
“All of your Omicron neutralizing antibodies in your blood are really coming from this process,” said Crotty. The B cells recognize the ancestral strain from the original vaccine and then those B cells mutate and bind to Omicron, even though they hadn’t yet encountered that variant before.
Structures called germinal centers, a source of generating B cell diversity, facilitate this evolutionary process.
“The presence of one virus showing up now may mean that there would be related viruses showing up in the future, and thus making a diversity of memory B cells would be quite helpful in recognizing viral variants in the future,” Crotty said.
Ongoing research by his lab into immune memory has implications for broader work in vaccine immunology. Currently, Crotty’s group is also working on candidate HIV vaccines and new vaccine strategies that may apply to many other diseases.
