Window to the Future
Bertozzi Reflects on the Potential of Bioorthogonal Chemistry
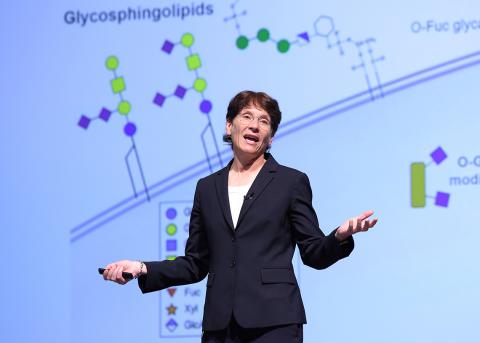
Photo: Chia-Chi Charlie Chang
What’s a chemist to do when her ambitions expand beyond the limits of her field? If the chemist in question is Dr. Carolyn Bertozzi, the answer is clear: invent a new type of chemistry.
“As abstract as that sounds, I had a very specific application in mind,” said the Nobel laureate and Stanford University academic at a Wednesday Afternoon Lecture Series (WALS) lecture earlier this year.
As a postdoctoral fellow in the early 1990s, Bertozzi was conducting basic science research in an immunology lab studying changes in patterns of cell surface glycosylation related to inflammation. The glycocalyx, or the pattern of sugar molecules on the cell surface, changes in response to diseases such as cancer. Bertozzi became interested in imaging these changes, and came up with a method that would require doing chemistry on live cells within live animals, and ultimately within human patients.
But her envisioned approach would require an entirely new kind of chemistry. She dubbed it “bioorthogonal”— chemistry between reagents that neither interact nor interfere with a biological setting.
Now an established field, bioorthogonal chemistry allows organic synthesis ordinarily performed in a lab to be performed in biological environments without affecting biomolecules or interfering in biological processes.
How could this be applied to Bertozzi’s immunology research?
“Could we image sugars on the surface of cells and use that to differentiate healthy and cancerous cells?” Bertozzi wondered.
More specifically, she envisioned tagging a specific molecule within the glycocalyx called sialic acid, which grows in a healthy pattern in healthy cells and overgrows in cancerous cells.
She theorized, “If we could [illuminate] sialic acid, could that differentiate tumor tissue from healthy tissue?” However, the technology to image sugars did not exist in the 90s.
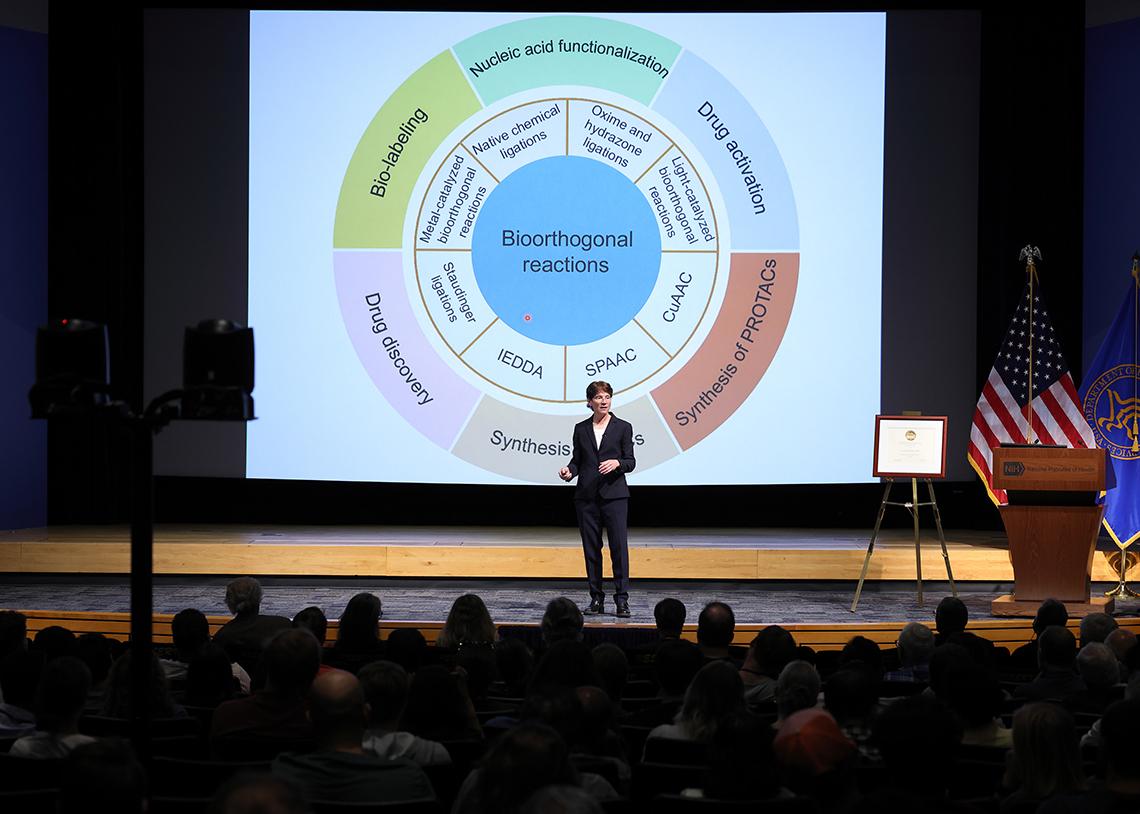
Photo: Chia-Chi Charlie Chang
This is where Bertozzi’s bioorthogonal chemistry came into play. If she could embed a chemical handle into the sugar and ensure the chemical reached the sialic acid, then maybe she could conduct additional chemistry and get the molecule to fluoresce.
In 2000, she devised a reaction that could be done on cells called a Staudinger ligation (an adaptation of another reaction called a Staudinger reduction). Bertozzi tested this reaction in a mouse model, injecting a precursor of sialic acid decorated with an azide group. After a few hours, the azido-sugar would be metabolized and incorporated into cell surface glycans. Finally, Bertozzi could use a Staudinger ligation to attach a phosphine probe, which became the flag on the cell surface.
This process was too slow, though. Another adaptation of an existing reaction, click chemistry, was the key to speeding it up. The original click chemistry uses a copper catalyst, which is toxic to living things. So Bertozzi developed a “copper-free” click chemistry to enable her to “click” a probe molecule onto the azido-sugar.
She demonstrated the success of this method in zebrafish embryos, labelling the developing organ systems with different colors and then imaging them. She likened it to “Turning on a new spotlight and shining it in a new place on a new thing.”
Bioorthogonal chemistry has continued to enable new discoveries, one of which disrupted a long-held standard of glycobiology knowledge. Textbooks generally agree that the cell surface contains two types of glyco-molecules—glycolipids and glycoproteins—but Dr. Ryan Flynn, a former member of Bertozzi’s lab, discovered that RNA is also glycosylated and presented on the cell surface.
“This is the kind of basic science tool that can allow you to open a window to a new type of molecule,” she said.
This basic science tool has also made possible exciting clinical translations. Bioorthogonal and click chemistry are now being used to make antibody-drug conjugates, vaccines, cell therapies and more.
Chimeric antigen receptor chemistry (CAR) T cell therapy, a new type of cancer immunotherapy, is one such example. A patient’s T cells are obtained from a blood sample and then genetically engineered in the lab to produ

Photo: Chia-Chi Charlie Chang
ce CARs, which enable the T cells to recognize and bind to specific cancer cell antigens. Technicians multiply the CAR T cells in the lab and then the cells are infused back into the patient, where they bind to the cancer cells and kill them.
This treatment does not work for everyone, however, and because it requires specialized facilities, most patients don’t have access. Bertozzi described the work of a biotech company formed by one of her former PhD students, Acepodia, that is generating “off the shelf” T cells from healthy donors and modifying them using bioorthogonal chemistry to target cancer cells.
In another cancer treatment application, Bertozzi described the work of biotech company Shasqi Pharmaceuticals, which is delivering chemotherapy treatments directly to cancer cells using bioorthogonal chemistry within the human body. This type of chemistry, called “click-to-release,” is a cousin of click chemistry.
“As one compound clicks on, another releases,” Bertozzi said, which makes it ideal for a prodrug—a pharmacologically inactive compound that is metabolized and activated inside the body. Shasqi has tested this method in a clinical trial with the chemotherapy drug doxorubicin. This drug causes unpleasant side effects with normal use because it can interact with healthy cells as well as cancer cells. When it is only activated in the tumor microenvironment with the click-to-release method, patients may experience fewer side effects.
Looking back to the basic science origins of bioorthogonal chemistry, she acknowledged its “many interesting applications that will hopefully translate into therapeutics.”
“We can’t predict the future, but I think bioorthogonal chemistry will be part of it,” she concluded.
Watch the archived lecture at
https://videocast.nih.gov/watch=55040.
