Discovery Paves Way for New Sight-Saving Therapy
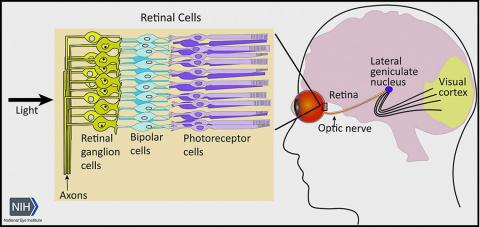
Photo: NEI
NEI-funded research offers a new path for preventing glaucoma vision loss. A form of gene therapy protects optic nerve cells and preserves vision in mouse models of glaucoma. The findings were published in Cell.
Glaucoma, a leading cause of visual impairment and blindness, results from irreversible neurodegeneration of the optic nerve, the bundle of axons from retinal ganglion cells that transmits signals from the eye to the brain to produce vision. Available therapies slow vision loss by lowering elevated eye pressure, but some glaucoma progresses to blindness despite normal eye pressure.
“Our study is the first to show that activating the CaMKII pathway helps protect retinal ganglion cells from a variety of injuries and in multiple glaucoma models,” said the study’s lead investigator, Dr. Bo Chen of the Icahn School of Medicine at Mount Sinai.
The CaMKII (calcium/calmodulin-dependent protein kinase II) pathway regulates key cellular processes and functions throughout the body, including retinal ganglion cells in the eye.
Using an antibody marker of CaMKII activity, Chen’s team discovered that CaMKII pathway signaling was compromised whenever retinal ganglion cells were exposed to toxins or trauma from a crush injury to the optic nerve. Administering the gene therapy to mice just prior to the toxic insult and just after optic nerve crush increased CaMKII activity and robustly protected retinal ganglion cells.
Among gene therapy-treated mice, 77 percent of retinal ganglion cells survived 12 months after the toxic insult compared with 8 percent in control mice. Six months following optic nerve crush, 77 percent of retinal ganglion cells had survived.
Similarly, boosting CaMKII activity via gene therapy proved protective in glaucoma models based on elevated eye pressure or genetic deficiencies.
“If we make retinal ganglion cells more resistant and tolerant to the insults that cause cell death in glaucoma, they might be able to survive longer and maintain their function,” Chen concluded.
