50 Years Later
Impact of 1971’s National Cancer Act Marked

Photo: NCI
This year marks the 50th anniversary of the passage of the National Cancer Act of 1971 (NCA), the landmark legislation that catalyzed breakthroughs in cancer research and care and improved the lives of the American people.
“The NCA advanced NIH’s mission to improve the public’s health through scientific discovery,” said NCI director Dr. Ned Sharpless. “The programs and advances enabled by the legislation accelerated improvements in cancer prevention, early detection and treatment over the decades that have dramatically reduced the burden of cancer in our country and far beyond.”
By 1970, cancer was the second leading cause of death in the U.S. and the number one health concern of the American people. Recognizing public anxiety and the need for more funding for cancer research, Mary Lasker and other advocates pushed for legislation that would substantially increase the country’s commitment to making advances against the deadly disease.
On Dec. 23, 1971, President Richard Nixon signed NCA into law, providing NCI with expanded authorities and responsibilities through development of a National Cancer Program.
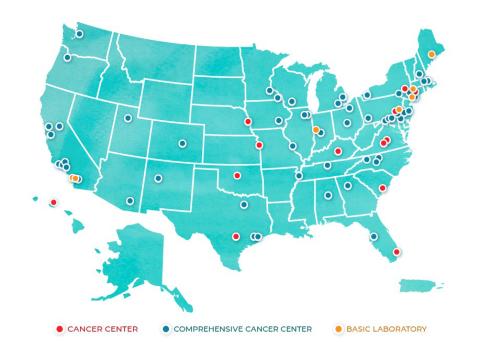
One of the most forward-thinking concepts to emerge was the modern-day NCI Cancer Centers Program, an anchor of the nation’s cancer research effort. NCA initially directed NCI to establish 15 new research facilities. There are now 71 NCI-Designated Cancer Centers in 36 states and the District of Columbia. They perform cutting-edge research and conduct practice-changing clinical trials, and many deliver state-of-the-art treatment throughout the nation, including in a number of underserved communities.

Photo: National Library of Medicine
NCA also established the foundation for a nationwide cancer clinical trials program. Many trials under this network helped establish standards of care and produced numerous treatment advances. Now known as the National Clinical Trials Network, the program encompasses trials at more than 2,200 sites internationally. Recent analysis found that over 40 years, findings from the clinical trials network have added 14 million years of life for people in the U.S.
NCI’s commitment that all people benefit from clinical trials also led to the creation, in 2014, of the NCI Community Oncology Research Program (NCORP). Through NCORP, more people, particularly those from underserved populations, have access to clinical trials and innovative studies of ways to prevent cancer and manage the short- and long-term effects of cancer and its treatments.
Another vital mandate of NCA was collection, analysis and dissemination of data that can provide insights into the state of cancer prevention, diagnosis and treatment. That directive prompted establishment of SEER (Surveillance, Epidemiology and End Results) in 1973.
SEER now covers 48 percent of the nation’s population, including 22 different geographic areas, and collects data on approximately 800,000 new cancer cases (without any personally identifying information) each year.
Recent SEER expansions focused on areas that include more people from underserved and ethnic/racial groups. Data from SEER allows researchers to perform population-based studies that identify new trends in cancer incidence. More recently, pilot studies were launched that link SEER data with information from commercial insurers to better understand trends in use of specific cancer drugs.
The NCA’s passage half a century ago has contributed to the consistent decline in the overall cancer death rate in the U.S., which has gone down 31 percent since its peak in 1991. A key contributor to that decline is progress in cancer prevention, including the HPV vaccine, comprehensive tobacco control programs that have slashed smoking rates, and effective screening options for colorectal and breast cancers.
Cancer treatment has also radically transformed. Although surgery, chemotherapy and radiation therapy are still mainstays of cancer treatment, therapies that target tumors with specific genetic alterations and immunotherapy, which unleashes a patients’ immune system on their cancers, are now standard treatments for a growing number of cancers.
NCA also opened the door to collaboration between other biomedical research fields, including a rich cross-fertilization between cancer and HIV/AIDS research. In 1981–just 10 years after the NCA was signed–the Clinical Center admitted one of the first patients with what would come to be known as AIDS. NCI’s expertise in retrovirology, immunology and drug development uniquely positioned the institute to make early advances in the fight against HIV/AIDS and AIDS-associated cancer. NCI scientists co-discovered HIV, showed that it was the cause of AIDS, developed the first blood test to prevent the spread of HIV by blood transfusion, and developed the first effective drugs to treat HIV, including azidothymidine (also called AZT or zidovudine).
Another unique aspect of the NCA was its mandate to establish two advisory groups: the National Cancer Advisory Board, a presidentially appointed panel of research experts and advocates that provides guidance to NCI leadership, and the President’s Cancer Panel, which reviews the National Cancer Program and submits an annual report on specific topics of importance (e.g., HPV vaccine uptake, clinical trials enrollment) to the President. Additionally, NCA made the NCI director a presidential appointee.
“The essence of the [NCA] was to provide not only more resources for cancer research, which it did, but also some protection from the bureaucratic processes that tend to envelop special initiatives,” explained Dr. Vincent T. DeVita, Jr., former NCI director (1980-1988). “[The protection came with] special authorities to allow the institute to operate with greater speed and flexibility, and special reporting lines to troubleshoot problems that might arise.”
As the NCA signing anniversary nears, NCI is committed to President Biden’s goal to “end cancer as we know it.” By building on 50 years of progress, advancing health equity, personalizing cancer care, embracing technological innovation, inspiring the next generation of diverse researchers and preparing for the challenges of the future, NCI believes it can reach this goal.
“I believe it’s within our power to deliver on President Biden’s call to action to end cancer as we know it,” said Sharpless. “And nothing will stop us from reaching that goal.”
Who are the people behind 50 years of progress in cancer research and care? Learn their stories at www.cancer.gov/news-events/nca50?cid=wl_ext_en_nihrecord_nca50.
