Injectables for Healing
Bioengineer Develops Ways to Repair, Regenerate Tissue

Dr. Karen Christman went into bioengineering to find novel ways to help patients, and she’s getting ever closer to that goal. While the therapies her lab developed are in preclinical or early-phase clinical testing, studies so far show hopeful results.
Christman’s lab at the Sanford Consortium for Regenerative Medicine in San Diego works with different types of biomaterials—both naturally derived and synthetic hydrogels—as well as nanoparticles to repair and regenerate tissue toward treating cardiovascular and other conditions. She discussed her research at a recent virtual meeting of NIH’s biomedical engineering scientific interest group.
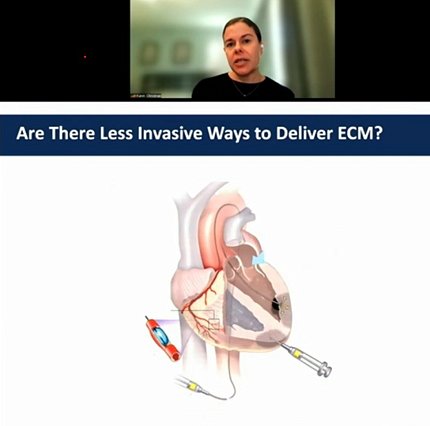
“Almost everything we do really is geared to developing minimally invasive therapies, [ones] that don’t require surgery and can be delivered either through intravenous injection, intramuscular injection or, in the case of the heart, via catheter,” said Christman, a professor of bioengineering at the University of California San Diego. Such modes of delivery, she said, mean less recovery time for the patient.
Healing the Heart
“We think a lot about how we can use biomaterials to create new environments in the heart that will stimulate healing,” said Christman.
Myocardial infarction (MI or heart attack) remains a leading cause of death around the world. Of those who survive, many experience negative left ventricular remodeling and don’t recover full cardiac function. There are no therapies that prevent this process, which leads to reduced pumping capacity and ultimately end-stage heart failure. Current treatment options for end-stage heart failure, such as heart transplants or left ventricular assist devices, are invasive and have other downsides.
In acute MI, cardiac inflammation degrades the extracellular matrix (ECM)—the body’s natural scaffold, or microenvironment for cells—which over time gets replaced with a dense collagen scar. Rather than infuse cells into such an abnormal environment that has diseased cues, Christman’s approach tries to recreate a more normal environment where cells can heal the heart.
“You can think of it as trying to create a healing wound inside of the heart as opposed to a chronic and inflammatory scar,” she said.
One of the first technologies her lab developed was an injectable myocardial matrix hydrogel derived from cardiac tissue from pigs. A catheter with a retractable needle delivers the hydrogel directly into the heart.
In animal models, injecting the hydrogel after MI affected multiple dysregulated pathways and showed increases in cardiac muscle, signs of new muscle formation and improved cardiac function.
This research led to a small phase-1 safety trial of the VentriGel injected into 15 patients between 2 months and 3 years after MI.

Photo: University of California San Diego
“The safety results alone were very exciting because—at least to our knowledge, while [there are] extracellular matrix patches, or ground-up powders that you apply for wound healing or surgical applications—nobody had translated an [ECM] hydrogel,” said Christman. “This was the first one, [not to mention] going into a very high-risk location like the heart.”
For the 15 patients, the procedure was well-tolerated. Heart symptoms improved while exercise capacity significantly increased. Another phase 1 study is planned for coronary artery bypass graft patients who cannot be fully revascularized.
Building on that success, Christman’s lab began looking at peripheral artery disease (PAD), for which there are no effective therapies. PAD leads to muscle atrophy and limb ischemia; severe cases lead to limb amputations.
“We thought we could use a similar strategy of having an extracellular matrix hydrogel to stimulate healing of the tissue and, in this case, try to treat ischemic skeletal muscle,” Christman said.
Preclinical work looks promising. Using a rat model, her lab resected part of the femoral artery and vein—causing a decrease in blood flow followed by inflammation—then injected the hydrogel.
“We got significant improvements with the skeletal muscle extracellular hydrogel in perfusion down to the foot,” said Christman. The treatment prevented muscle atrophy and spurred regeneration, with significant increases in muscle stem cells.
Improving Women’s Health
Recently, Christman’s lab also began focusing on women’s health. Collaboration with a physician-scientist with expertise in female pelvic medicine led to research on pelvic floor disorders, caused by injury to pelvic skeletal muscles commonly from vaginal birth. There are rehab exercises, compensatory devices and surgeries, said Christman, but “there’s nothing that is treating the muscle injury and trying to restore muscle function.”
In preclinical testing, when injecting either saline or skeletal muscle ECM hydrogel, “we get a greater restoration to healthy levels with the hydrogel,” she noted. The injection prevented atrophy, decreased fibrosis and showed signs of skeletal muscle regeneration.
“We’re hopeful we can actually accelerate this in the patient,” said Christman, “to see if we could use this in women showing signs of pelvic floor muscle dysfunction and prevent them from getting prolapse.”
Other Applications?
With heart patients, injection would take place weeks or even months after the MI, but Christman also seeks to treat patients at the time of infarction.
“There is a risk injecting anything into patients within the first month,” she said, “because going in there with the needle, poking in the myocardium, could set off arrhythmias” or ventricular ruptures.
One way in would be through balloon angioplasty, infusing the material in short bursts distal to the balloon. Preclinical results suggest accelerated vascular healing.
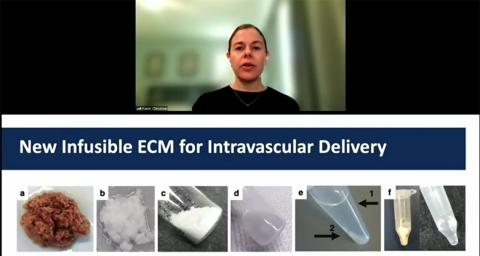
Christman also continues to research new applications for ECM therapies and her lab has developed a new way to deliver decellularized ECM through intravenous delivery.
“This has applications beyond the heart, because there are a lot of diseases and conditions where you have inflamed, leaky vasculature,” Christman said.
Her lab has begun to study the potential of this infusible ECM to treat traumatic brain injury, pulmonary arterial hypertension and acute respiratory distress syndrome.
“We now think we have a therapeutic that can really be more broadly used across multiple conditions where you have leaky vasculature and inflammation,” Christman said. “We’re quite excited about the potential of them to reduce vascular leakage, accelerate vascular healing, be immunomodulatory and pro-survival.”
Studies also show the ECM hydrogels can improve cell survival and engraftment and may enhance retention and delivery of other biologics and medications.
“Extracellular matrix hydrogels can be a powerful strategy for regenerative engineering,” concluded Christman. “I think this really opens up the potential of using these hydrogel technologies for a lot of other indications.”
