Researchers Create 3-D Model for Rare Neuromuscular Disorders
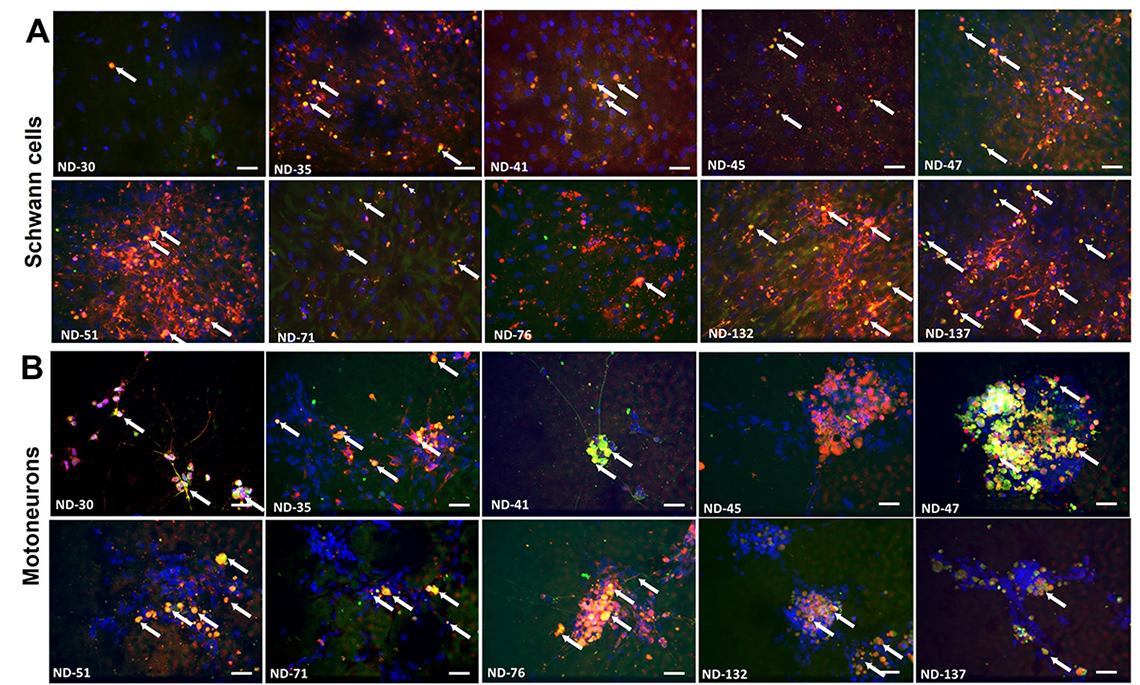
Photo: Wiley-VCH
A scientific team supported by NIH has created a tiny, bioengineered 3-D model that mimics the biology of chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy, a pair of rare, devastating neuromuscular diseases. The researchers used the organ-on-a-chip, or “tissue chip,” model to show how a drug could potentially treat the diseases. They provided key preclinical data for a drug company to submit to the Food and Drug Administration to get authorization for testing in a clinical trial.
Investigators are exploring the use of tissue chips for testing candidate drugs and modeling diseases. Designed to support living human tissues and cells, tissue chips mimic the structure and function of human organs and systems, such as the lungs, heart and liver.
This work provides one of the first examples of scientists using primarily tissue chip data for an FDA investigational new drug application to test the efficacy of a candidate drug in people with rare diseases. The drug company Sanofi started recruiting participants into a phase 2 clinical trial in April 2021. The drug was tested for safety previously and approved by the FDA for a different indication.
The tissue chip research was led by Hesperos, Inc., a company partially funded by a small business innovation research grant from NCATS.
In these neuromuscular diseases, the immune system makes proteins called antibodies that damage nerve cells and slow down messages moving from the brain to the muscles, making it hard for people to move their limbs.
Dr. James Hickman, chief scientist at Hesperos, and his colleagues described their model in Advanced Therapeutics. Their tissue chip consisted of two cell types: motoneurons (which transmit messages from the brain to muscles) and Schwann cells, which help the signals move more quickly.
The researchers showed that exposing the cells to blood serum from people with these rare diseases caused a shower of immune system antibodies against the cells. This made the motoneuron signals move more slowly. After treatment with TNT005, a drug that blocks the immune system reaction, the cells and the message speed returned to normal.
This study could open the door to developing new therapies for other rare diseases by establishing a new avenue for repurposing existing drugs.
