Two Diabetes Drugs Outperformed Others in Clinical Trial
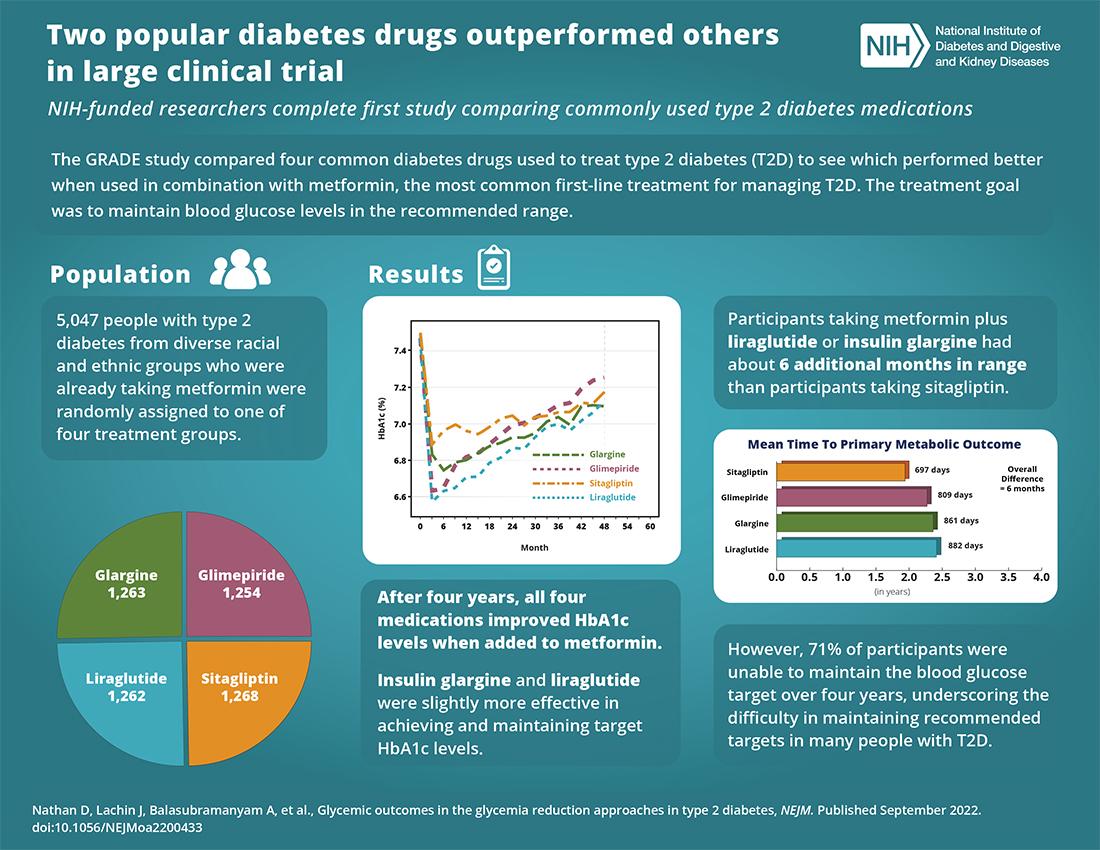
Photo: niddk
In a large, NIDDK-funded clinical trial comparing commonly used type 2 diabetes medications, researchers found that insulin glargine and liraglutide performed the best of four medications FDA-approved to maintain blood glucose levels in the recommended range.
All four medications evaluated were added to treatment with metformin, the first-line drug to treat type 2 diabetes.
More than 37 million Americans have diabetes; approximately 90 to 95 percent of them have type 2 diabetes. People with diabetes who keep their blood glucose levels in the near-normal range generally have a much lower risk of developing diabetes complications such as nerve, kidney and eye diseases. Most people with type 2 diabetes require more than one medication to control blood sugar levels over time.
While there is general agreement among health care professionals that metformin combined with diet and exercise is the best early approach in diabetes care, there is no consensus on what to do next to best keep high blood glucose in check.
Launched in 2013, the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness (GRADE) Study, was conducted at 36 U.S. study centers. Results were published in the New England Journal of Medicine.
The study enrolled 5,047 people with type 2 diabetes from diverse racial and ethnic groups who were already taking metformin. Participants were randomly placed into 1 of 4 treatment groups. Three groups took metformin plus a medicine that increased insulin levels, sitagliptin, liraglutide or glimepiride. The fourth group took metformin and insulin glargine U-100, a long-acting insulin.
After an average of 4 years of follow-up, the study found that participants taking metformin plus liraglutide or insulin glargine achieved and maintained their target blood levels for the longest time compared to sitagliptin or glimepiride. Treatment effects did not differ based on age, sex, race or ethnicity. However, none of the combinations overwhelmingly outperformed the others.
Dr. Henry Burch, NIDDK’s project scientist for GRADE, said the study “is an integral step toward precision medicine for diabetes care, as these results can now be used in the decision-making process for each individual patient in light of their levels of glucose control, how well the medications are tolerated and the person’s other health considerations.”
