Whitehead Recounts Journey to Developing Dengue Vaccine

Anyone who lives in, or travels to, areas where dengue fever is prevalent knows the fear of getting bitten by a dengue-carrying mosquito. The dengue virus, spread by a certain mosquito species in tropical climates, can cause severe illness. If not treated properly, it can be deadly.
Dr. Stephen Whitehead has been developing vaccine candidates for dengue and other arboviruses at NIH for 25 years. One of those vaccines recently completed a Phase-3 clinical trial in Brazil.
Whitehead, a senior investigator in the Arbovirus Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), discussed efforts toward developing a dengue vaccine at this year’s NIH Research Festival, where he delivered the Philip S. Chen Jr. Distinguished Lecture on Innovation and Technology Transfer.
Some arboviruses (arthropod-borne viruses), such as West Nile and Zika, have short and sporadic outbreaks, making them difficult to study, he said.
“This low disease incidence makes it very difficult to do efficacy trials for vaccines,” Whitehead noted. “By the time you have a vaccine candidate ready and proceed to clinical testing, the virus has moved on; it’s no longer circulating. But dengue is different.”

There are more than 390 million dengue infections each year. The virus is endemic in more than 100 countries. Some 3 billion people live in an area of frequent or sporadic outbreaks across Africa, Central and South America and Asia.
“Dengue is both an established disease and an emerging and re-emerging disease,” Whitehead said.
Symptoms of a dengue infection can include fever, vomiting, rash, lethargy and, in more severe cases, shock, hemorrhage and respiratory distress.
“The case fatality is probably less than half a percent, but that is with proper case management,” he said.
In developing a vaccine, an envelope protein on the outside of the virus particle is the main target of neutralizing antibody. The challenge, though, is that dengue consists of four different serotypes. A person can get infected with more than one serotype throughout their life.

Photo: Marleen Van Den Neste
Exposure to each type elicits antibodies that provide natural immunity. But that can be a double-edged sword.
“Preexisting immunity to one dengue serotype is the greatest risk factor for more severe disease when you get your second infection,” Whitehead said. That incoming virus from a different serotype can be bound by antibodies that aren’t effectively neutralizing, which ultimately can enhance infection and increase the viral load.
“This is why partial immunity to dengue can be dangerous,” he said. Therefore, “we need a vaccine to provide effective tetravalent immunity.”
In the streets of Bangkok, millions of adults are walking around immune to all four dengue serotypes due to sequential exposure throughout their lifetime. “This is what natural immunity looks like,” Whitehead said. But what about people with no previous exposure? “Can we safely induce this immunity in children?”
Whitehead set out on a long journey to develop a live, attenuated dengue vaccine. Live vaccines tend to induce lifelong immunity, can be highly immunogenic with just one dose and they’re cost-effective to produce.

Photo: Marleen Van Den Neste
“For live vaccines, infection is required for immune stimulation,” Whitehead explained. “For an effective, live, tetravalent vaccine, all four serotype components need to replicate. That’s the foundation for developing the NIH vaccine.”
Currently, there are two commercially available dengue vaccines. Sanofi’s vaccine shows efficacy as high as 93% against serious dengue but it’s recommended only for those who have had a previous infection and has not proven effective for certain serotypes. Takeda’s vaccine is also not for use in ‘dengue-naïve’—haven’t previously been infected—children. It too does not effectivelyprotect against all four strains.
“We worked for 25 years, moving hundreds of different virus constructs through preclinical studies, most of them made in the laboratory,” Whitehead said. They then put forward the best candidates in monovalent clinical studies.
“Why our vaccine works is because we went and tested each serotype individually in human Phase-1 trials.” That took about a decade. “We wanted to make sure [each] was going to work so we could come up with this final tetravalent formulation.”
They conducted 31 different trials to tweak this vaccine. A winning candidate was tested in Thailand and Bangladesh that has proven uniformly immunogenic, even in dengue-naïve subjects; it’s safe in older adults up to age 70 and in infants and children. And it’s protective as early as 30-days post-vaccination, which is important for protecting those planning to travel to dengue-endemic regions.
The vaccine study in Bangkok showed high antibody responses in children who, prior to vaccination, had little or no immunity. “The immune profile of these children now looks very similar to what you see after natural infection in adults,” Whitehead noted. And in adults, “We have 100% protection demonstrated in our controlled human-infection model.”
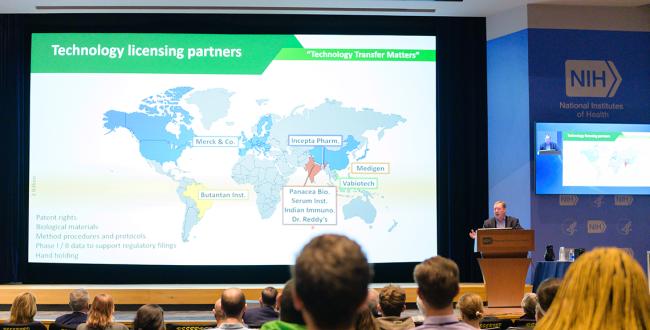
Photo: Marleen Van Den Neste
The next step was technology transfer. NIAID licensed this technology—including the patent rights, biological materials and methods, and data to date—to multiple companies to develop in the U.S. and abroad.
The Phase-3 study in Brazil, conducted in more than 16,000 participants at 16 clinical sites across Brazil by Instituto Butantan, showed 88% efficacy overall against severe dengue for two years. After more than three years, a single dose still showed 67% overall efficacy against any severity of dengue, including good protection even among the youngest children. Overall, Whitehead noted, the NIH vaccine outperforms the two others currently on the market.
One caveat is that two of the four serotypes were not circulating in Brazil during the study. Merck, Inc., which would distribute the vaccine in the U.S., China and across Europe, is currently running its own trial and found one dose elicited neutralizing antibodies for all four serotypes.
Another Phase-3 trial began this summer with DengiAll, the brand name of the NIH vaccine produced by Panacea Biotec in India. The trial will enroll 10,000 healthy Indian adults, ages 18-60, at 19 clinical sites. Whitehead expects stellar results from this trial when data becomes available.
“How did we get here? By technology transfer,” said Whitehead. “What started on the bench, moved to the bedside and then [we] are moving into implementation.”
