Senators Visit NIH, Take Home Tech

Photo: Chia-Chi Charlie Chang
A bipartisan contingent of U.S. senators and staff members visited NIH on May 17 for science briefings, a lab tour and biotech demonstration. The group included Sen. Tammy Baldwin (D-WI), Sen. Roy Blunt (R-MO), Sen. John Boozman (R-AR), Sen. John Cornyn (R-TX), Sen. Dick Durbin (D-IL), Sen. Roger Marshall (R-KS), Sen. Lisa Murkowski (R-AK) and Sen. Chris Van Hollen (D-MD). Many are members of the appropriations committee.
NIH director Dr. Francis Collins and NIAID director Dr. Anthony Fauci welcomed the group at the Vaccine Research Center. A tour of a VRC lab, a demonstration of Rapid Acceleration of Diagnostics (RADx) technology, a briefing on mental health amid the pandemic and a discussion of the potential “ARPA-H” were packed into the afternoon. Senators and staff members were separated into two groups to facilitate occupancy in small spaces.
For the lab tour, VRC director Dr. John Mascola; VRC deputy director Dr. Richard Koup, who is also chief of the Immunology Laboratory; and Dr. Nancy Sullivan, chief of the biodefense research section, joined in a discussion about vaccine development.
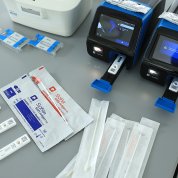
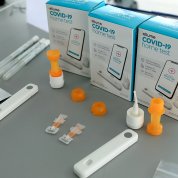
In a tent erected not far from the VRC, NIBIB director Dr. Bruce Tromberg provided an overview of RADx and walked the delegation through several technologies including Covid at-home tests, point-of-care tests and lab tests. [See RADx Tech Tent Show below] He highlighted efforts developed via RADx over the past year and showed a large sampling of new tests and products from 32 different companies. Each senator and staffer received a box of two at-home tests they could take with them.

Photo: Chia-Chi Charlie Chang
In a large Porter Bldg. conference room, NIMH director Dr. Josh Gordon and deputy clinical director Dr. Joyce Chung discussed mental health and Covid-19. Gordon provided an overview, with Chung presenting on intramural research efforts, collaborations and preliminary findings on the impact of the pandemic on mental health.
Collins briefly discussed ARPA-H, a potential new health research component devoted to scientific breakthroughs that would be housed within NIH. President Joe Biden proposed creating ARPA-H in a recent speech to Congress. Collins also talked about what the senators would see during their visit, which included several presentations on NIH’s multifaceted response to the Covid-19 pandemic.
Afterward, on social media, Blunt posted a message: “Thank you to National Institutes of Health (NIH) Director Dr. Francis Collins & researchers for giving us a terrific tour & presentation of their latest work to save lives. Because of medical researchers’ ingenuity & drive, we are on the edge of finishing the fight against Covid & on a faster timeline than we thought possible a year ago.”