‘Mini-Gut’ Makers
Pioneer in Field Describes Industrious Organoids

Photo: Hubrecht institute
Mini-guts. Organoids. It sounds like something out of science fiction, but these terms are part of researcher Dr. Hans Clevers’s everyday vocabulary. And these innovations are revolutionizing the way we treat cancer and other diseases.
Clevers, who is an M.D.-Ph. D. professor of molecular genetics at Utrecht University and principal investigator at Hubrecht Institute in the Netherlands, presented an overview of his research in a lecture titled “Organoids to Model Human Disease.”
Described by others as a pioneer in the field of organoids, Clevers and his team stumbled across their discoveries “more or less by chance,” he admitted.
First, Clevers spent some time studying a family of signaling molecules known as the Wnt pathway, which are important for immune response, as well embryonic development and tissue repair. Most importantly to his work, he learned, “Wnt in the gut is a driver of normal and cancerous stem cells.”
Stem cells in the gut? Turns out, most tissues in the human body contain a small number of stem cells, which replace other mature cells that have reached the end of their life cycle.
Inside Crypts
In the intestines, for example, stem cells originate from specialized cells in the epithelium (which form the lining of the digestive tract as well as our skin cells). The intestinal epithelium contains many types of cells, including tiny hair-like structures called villi, which help absorb nutrients from the food we eat, and crypt cells, which sit between the villi and churn out stem cells.
Stem cells are “born” in these crypts and embark on a 2-day journey to the surface. The cells divide and proliferate as they travel. As they exit the crypt, they differentiate into one of about 10 different intestinal cell types. Then these newly determined cells travel up the neighboring villi (which takes another 5-6 days) and onward to their destination.
So, where does the Wnt pathway come into play? Clevers made a groundbreaking discovery in a family of genes involved in the Wnt pathway called TCF, or t-cell factor. TCF regulates the Wnt pathway by deciding which genes are expressed—and those genetic instructions inform stem cells for tissue repair.
When Clevers was studying one specific factor, TCF4, and its role in colon cancer, he found that malfunctions in the TCF4 gene can cause mutations in stem cells. The malfunctions lead to abnormal cell formation and eventually to development of carcinomas.
DIY Crypts?
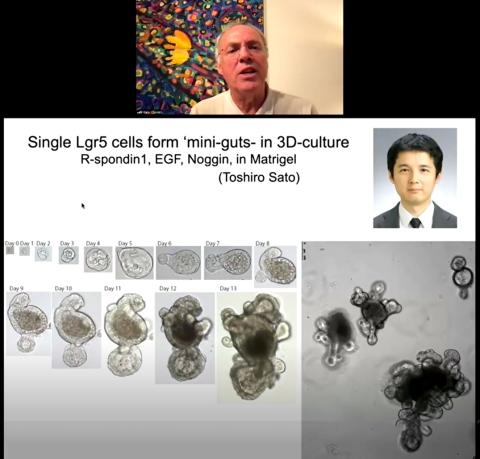
When Clevers and his team grew some of these intestinal stem cells in the lab, they made another startling discovery. They expected the stem cells to replicate in the petri dishes and create more stem cells, but instead, “we got epithelial structures,” Clevers said.
Given an ideal mix of growth factors, the stem cells had multiplied and differentiated, self-organizing into “crypt equivalents,” populated with stem cells as well as the other cell types from the primary epithelium. The stem cells “apparently remember how to build a complete version of the tissue that it [came] from,” Clevers realized.
These tiny structures were so similar on a cellular level to the intestine that the researchers dubbed them “mini-guts.”
Mighty Organoids
The intestinal organoids were also exciting because Clevers quickly realized they had incredible therapeutic and research potential. They can survive in culture for years without signs of aging. As a final test for the organoids, Clevers and his collaborators grew intestinal organoids from mouse stem cells and transplanted the organoids into the colons of mice that had inflammatory bowel syndrome (IBS).
The organoids acted like “a living band-aid,” said Clevers, by patching over the colon lesions and “fully integrating into the gut tissue.” This technology is now being studied in humans with IBS.
Gut stem cells are the most active stem cells in the human body (the epithelium replaces its cells every 5-7 days), but researchers can also grow organoids from stem cells in other less-proliferative organs.
The procedure is similar regardless of the type of tissue you are trying to grow, Clevers said. The process begins with a tissue sample, which is ground up and put in a medium containing a mixture of growth factors, hormones and other molecules that specific tissue needs to grow. All the cells die, except for the epithelial cells. They contain the stem cells that will regenerate the different types of cells that make up the tissue and ultimately produce organoids.
This technique is used to grow a wide variety of organoids, from pancreas to salivary glands and everything in between. Organoids can also be grown from diseased and cancerous tissue, Clevers said, where “you can measure the drug response in vitro [in a lab-grown organoid] and then show…the patient also respond to the [same] drug.”
Multi-Purpose Organoids
In other cases, organoids can be used to study the origin of cancer. Clevers and his colleagues used intestinal organoids to study a genotoxic form of Escherichia coli called pks E. coli. This common bacterium is found in the microbiomes of about 10 percent of the global human population.
Pks+ E. coli contains an extra region of DNA (the pks region) that codes for colibactin, a compound that creates breaks in the host cells’ DNA and can lead to new mutations as the DNA repairs itself.
In the study, pks+ E. coli induced DNA damage in healthy intestinal organoids. The researchers also exposed organoids to pks+ E. coli for 3 months to mimic chronic exposure and then sequenced the DNA of some of the organoids’ cells. The team found a unique pattern of mutation that is also seen in about 15 percent of colorectal cancer patients.
Based on this new research, pks+ E. coli could be considered a mutagenic/carcinogenic bacterium, which may inform preventive measures for patients who are predisposed to colon cancer.
Organoids are also useful in studying infectious diseases.
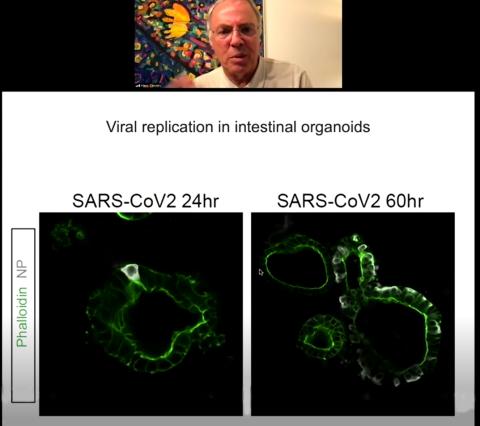
Clevers collaborated with a team in Rotterdam back in the beginning of the pandemic to study Covid-19. Very little was known about the virus at that time; the researchers wanted to learn more about where and how the virus replicated and what genes might be good drug targets.
Clevers’s intestinal organoids came in handy to answer those questions. He and colleagues demonstrated Covid replication in gut organoids and studied some of the genes that are crucial to the virus’s infectious cycle. They even identified the transmembrane protein TMPRSS2 (a protein that Covid uses to enter gut cells) as a potential drug target.
Clevers’s Covid research with organoids was especially exciting because, he said, “you can essentially read out in human organoids what the virus needs to propagate.
“A large number of studies now are validating organoids as an avatar of patients,” he concluded. Possibilities for the technology are exciting and this is just the beginning.
View an archived recording of the lecture at https://videocast.nih.gov/watch=44246.
